New method estimates sulfuric acid emissions from fired heaters
When a sulfur-bearing fuel is burned, essentially all the sulfur is initially converted to SO2, the primary form of sulfur emissions from combustion equipment. A small portion of the SO2 converts to SO3 and then some of this SO3 forms sulfuric acid aerosol.
We have documented calculation procedures that estimate the sulfuric acid emissions from the stacks of fired heaters and boilers in petroleum refineries and petrochemical plants burning typical liquid and gas fuels.
These procedures do not apply to other combustion equipment in these plants such as flares, incinerators, gas turbines, diesel engines, and FCCU regenerators. The procedures are, however, applicable to fired heaters and boilers in other process industries.
In June 1995, the US Environmental Protection Agency (EPA) modified the reporting requirements for sulfuric acid emissions under Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA).1 Only aerosol forms must be reported, and nonaerosol forms are excluded.
EPA defines sulfuric acid aerosols to include mists, vapors, gas, fog, and other airborne forms of any particle size. In November 1997, EPA issued a guidance document on estimating sulfuric acid emissions from numerous sources including combustion equipment burning sulfur-containing fuels.2
Conversion of SO2 to SO3
The amount of SO2 in the flue gas that is converted to SO3 is a function of fuel sulfur content, excess oxygen in the flue gas, flue-gas temperature, the catalytic effect of fuel trace metals (e.g., vanadium, sodium, etc.), and the type of combustion unit.
EPA indicates that 1-5% of the fuel sulfur can oxidize to SO3 for liquid fuels in fired heaters and boilers.2 3 The EPA guidance document provides a table of expected SO3 concentrations in oil-fired units as a function of the fuel sulfur content and excess air in the operating unit.
Based on values in the EPA table, SO2 conversion to SO3 is less than 3% and is relatively constant at greater than 1 wt % sulfur in the fuel oil at constant excess air. Conversion to SO3 decreases as the excess air falls from 25% to 5%.
For fuel with less than 1 wt % sulfur, conversion of SO2 to SO3 begins to increase rapidly with decreasing sulfur content. This response is consistent with data in the literature in which a conversion as high as 13% was measured for a fuel oil with about 0.1 wt % sulfur burned at 25% excess air.4 5
One can expect an increase in conversion at very low sulfur content because the ratio of oxidant to SO2 is greater.
If other data are not available, assume that 3% of the fuel sulfur is converted to SO3 for fired heaters and boilers that are firing liquid fuels. These units generally burn a residual fuel oil with greater than 1 wt % sulfur.
Since fuel gases burned in fired heaters and boilers typically have low concentrations of sulfur compounds (50-200 ppmv H2S), a greater amount of SO2 may convert to SO3 based on the data reported for burning fuel oil.
Fig. 1 shows the results from a series of tests conducted on two refinery fired heaters burning fuel gas. In these tests, the H2S in the fuel gas varied from about 100 ppmv (0.024 wt % S) to about 1,200 ppmv (0.30 wt % S).
The fired heaters operated with stack temperatures of 400-500° F. and stack excess oxygen concentrations between 3% and 4% dry O2 (about 15-20% excess air). These conditions approximate those frequently found in well-operated fired heaters and boilers.
In the absence of any other data, one should use Fig. 1 to estimate the conversion of SO2 to SO3 for fired heaters and boilers burning fuel gases. There is some uncertainty, however, in the higher conversion rates at the low fuel sulfur levels because the trend is based on only one test below 400 ppm.
Conversion of SO3 to H2SO4
Some of the SO3 in the flue gas combines with water vapor in the combustion products to form sulfuric acid. The amount of sulfuric acid formed depends on the stack-exit temperature and flue-gas water concentration.
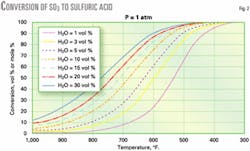
Fig. 2 gives the conversion of SO3 to sulfuric acid as a function of flue-gas temperature and water concentration. Fig. 2 assumes that the reaction reaches equilibrium; this is reasonable because the residence time in the stacks of most fired heaters and boilers is on the order of 1 sec.
We derived the curves using a calculation procedure based on commercial process simulation software. Fig. 2 shows that SO3 conversion to H2SO4 is about 93% at typical fired heater stack conditions (500° F., 10 vol % H2O).
Estimating sulfuric acid emissions
When a sulfur-bearing fuel is burned, 100% of the fuel sulfur is converted to a distribution of SO2, SO3, and H2SO4. This information is needed to calculate sulfuric acid emissions:
Fuel properties, including sulfur content.
Flue-gas conditions, including water concentration and stack temperature.
Following is the calculation procedure:
SO3 = (SO2 to SO3 % conversion/100) x SO2
Sulfuric acid = (Percent conversion/ 100) x SO3
- Calculate the final SO3 by subtracting the portion converted to sulfuric acid.
The accompanying boxes show two sample calculations.
References
- EPA, "Sulfuric Acid: Toxic Chemical Release Reporting: Community Right-to-Know," Final Rule, 60 FR 34182, June 30, 1995.
- EPA, "Emergency Planning and Community Right-to-Know Act—Section 313. Guidance for Reporting Sulfuric Acid (acid aerosols including mists, vapors, gas, fog, and other air borne forms of any particle size)," EPA-745-R-97-007, November 1997.
- EPA, "Compilation of Air Pollutant Emission Factors (Ap-42), Volume I: Stationary Point and Area Sources, Section 1.3 Fuel Oil Combustion," pp. 1.3-1 to 1.3-35.
- Rendle, L.K., Wilson, R.D., and Wittingham, G., "Fire Side Corrosion in Oil-Fired Boilers," Combustion, August 1959, pp. 30-42.
- Exley, L.M., "A Practical Review of Residual Oil Firing Problems and Solutions," Combustion, March 1970, pp. 16-23.
The authors
I. D. Crane retired from ExxonMobil Research & Engineering Co., Fairfax, Va., in 2000 after more than 35 years of developing, designing, and optimizing fired heat transfer and combustion equipment. He was head of the combustion emissions estimating and control group. Crane holds a BS and MS in mechanical engineering from Clarkson University, Potsdam, NY, and is a licensed professional engineer.
R. D. Springer is a senior technologist with OLI Systems Inc., Morris Plains, NJ. Previously he worked for Exxon Research & Engineering Co. He has over 20 years' experience in applying thermodynamics to solve industrial problems. He holds a BS in chemical engineering from the University of Cincinnati and an MS in chemical engineering from the New Jersey Institute of Technology.
J. H. Siegell is an advanced engineering associate with ExxonMobil Research & Engineering Co. where he heads activities on estimating and controlling air toxics, hydrocarbons, and odorous emissions. He has over 20 years' industrial experience in fundamental and applied research, and technical support to refineries and chemical plants. Siegell holds a BE and ME in chemical engineering from the City College of New York and a PhD in chemical engineering from the City University of New York. He is active on several API committees and currently leads the API smart leak detection and repair (LDAR) program.