Understanding how distillation trays work
Norman P. Lieberman
Process Improvement Engineering
Metairie, La.
A distillation tray is used to mix vapors and liquids together. The better the mixing, the more efficiently the tray works. Tray efficiency also depends on two other factors: how well the droplets of entrained liquids are separated from the vapor's flow rising from the tray deck and how well the bubbles of vapor are separated from the liquid flowing down from the tray deck through the downcomer. Regarding tray fractionation efficiency, the latter factor is of secondary importance. The former factor-i.e., entrainment-is of primary importance. It's what Stokes's law is all about.
Entrainment, tray efficiency
As a young man, I would often dream of meeting my childhood heroes: Nikola Tesla, Daniel Bernoulli, Isaac Newton, Sir George Stokes.
On one occasion, I dreamed that I'd passed on to my eternal and heavenly home and was welcomed by my famous colleagues and fellow scientists.
"Welcome, Norman, to the Eternal Life," Stokes announced to me cordially. "Can we answer any questions?"
"Well, yes, Mr. Stokes. I'm interested in entrainment. On earth, this is a big problem affecting fractionation efficiency. I assume it's also a problem here in the Eternal World. Can you tell me, then, what factors increase entrainment?"
Stokes explained several main factors that impact entrainment. These include:
• Droplet size. The smaller the drop, the greater the rate of entrainment.
• Viscosity of the continuous phase. High viscosity promotes entrainment.
• Density difference between the vapor and liquid phases. Reduced density difference promotes entrainment.
• Velocity. Higher velocities promote entrainment as well.
"I've actually reduced all these factors into a simple equation," Stokes explained with a smile. "I call it Stokes's law."
Mathematically, we say entrainment is proportional to V x (DV ÷ DL)0.5, where V = velocity of vapor phase, DV = density of vapor phase, and DL = density of liquid phase.
"But Mr. Stokes," I objected, "how about viscosity and the droplet size?"
"Well, Norman, the droplet size is usually unknown. Viscosity of a vapor phase is usually small, and I normally neglect it. By small, I mean less than 0.3 cp."
"That's all quite interesting. But if I could take a single step to suppress entrainment, which is a source of evil on earth, what would you recommend?"
"The best option would be to avoid high localized velocities of the vapor phase. Norman, I would also…"
"Wait a bit, Mr. Stokes!" Nikola Tesla interrupted. "Mr. Lieberman has arrived in Eternity prematurely. He's being deported at once, back to his earthly home in New Orleans."
Liquid entrainment, fractionation
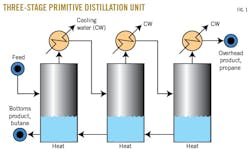
Originally, the process of distillation did not utilize trays. It relied on pots (Fig. 1). The pots, which were very large, completely separated the liquid phase from the vapor phase before the liquid and vapor left each pot. That is, there was no entrainment.
Each pot was an individual separation stage. If the separation of vapor and liquid phases was perfect, the pot had a separation, or stage efficiency, of 100%. The more pots that were arranged in series, the greater the degree of separation that could be achieved between the two products.
In our case, we are trying to separate propane and butane as efficiently as possible. To avoid the expense and mechanical complexity of dozens of these pots, the distillation column was invented about 180 years ago.
Each tray is intended to represent a pot, or at least a portion of a pot. If the tray really is working as well as a pot, it's said to have 100% tray fractionation efficiency. If the tray is only working half as well as the pot, it is said to have a 50% tray efficiency. The usual cause of low tray efficiency is entrainment due to excessive vapor velocity.
As the distance between the liquid level on the tray below and the tray above gets smaller, it takes less vapor velocity to blow the liquid from the lower tray up to the tray above.
For example, let's say that I'm separating propane from butane. My propane product (i.e., LPG in 5-gal containers sold in Walmart) has a maximum butane spec of 2%. If the vapor rate flowing up through a tower increases, the droplets of liquid from a lower tray will be entrained and blown up to the tray above. As the liquid in the lower tray is rich in butane, the entrained droplets will increase the butane content of the upper tray. Eventually, as the entrained droplets reach the top tray, the overhead LPG propane product will be contaminated with butane. Thus, fractionation efficiency is reduced.
Foam
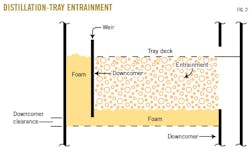
The fluid on a distillation tray deck is not actually liquid but foam or froth. The vapor bubbling up through the holes on the tray deck mixes with the liquid from the tray above to form foam. The foam overflows the low dam into the downcomer (Fig. 2). If the downcomer is too small, however, the foam fills up the downcomer and backs up onto the tray above it. The foam level on the tray then increases. As the froth or foam level increases on the tray, so does the height of the entrained liquid, or spray height.
When the spray height from the tray below reaches the tray above, tray fractionation efficiency becomes worse due to entrainment.
Reflux
More reflux improves tray fractionation efficiency. The trays work harder and better due to the increased vapor and liquid traffic on the tray decks.
The problem is that the reflux comes from the vapor generated by the reboiler. If there is too much vapor flow through the tray decks, then the vapor velocity may become excessive. According to the law of Mr. Stokes, my mentor in the Next World, this will cause excessive spray height, or entrainment.
It's rather like exercise, where too much running actually can be bad for one's health. Too much reflux and too much reboiler heat are going to make fractionation efficiency worse rather than better due to entrainment.
Of course, you can't just increase the reflux rate without more reboiler duty because the reflux is just condensed vapor generated from the reboiler that rises through the trays, through the overhead condenser, and into the tower's reflux drum.
Downcomer backup
Downcomers often back up onto the tray above, causing a high froth or foam level on the tray and a consequent entrainment of droplets of liquid to the tray above the tray draining into the downcomer.
Usually, it's not the downcomer itself that has too small an area. It's more typically because the downcomer is too short. The height of foam in the downcomer is mainly a consequence of the pressure drop of the vapor flowing through the tray above the downcomer (Fig. 2). As the vapor flows through this tray, it undergoes a pressure drop due to the restriction of the caps, or orifices, on the tray deck and the weight of the liquid on the tray deck.
In other words, the pressure of the vapor below the tray pushes up the foam height in the downcomer. Once the downcomer becomes full of foam, the foam backs up onto the tray above that downcomer. The extra weight of foam on the tray deck then increases the pressure drop of the vapor through that tray deck, which in turn increases the force pushing up the liquid out of the downcomer.
The problem feeds upon itself until the tower's trays above the tray where the problem began start to operate in a state called "fully developed flood."
A tray downcomer can also fill with foam because it can't drain out of the downcomer fast enough. This common problem is most often caused by a too-small downcomer clearance (Fig. 2). A reasonable downcomer clearance is usually 2-3 in. Any clearance less than 1.5 in. is likely to result in downcomer flooding and liquid backup on the tray above.
Unfortunately, if the downcomer clearance is greater than the tray's outlet dam height, the downcomer will also back up onto the tray above. This is called unsealing of the downcomer. Vapor will blow into the bottom of the downcomer and displace the liquid flow, resulting in fully developed flood.
As you read this section, keep in mind that the downcomer is full of frothy foam and not the denser liquid phase. Hence, this foam is rather easily displaced by the vapor flowing up through an unsealed tray downcomer.
Tray efficiency
Certainly, tray fractionation efficiency is reduced by entrainment, which is also called jet flood. Most tray efficiency problems, however, are caused by tray decks that are not level. The liquid on the tray deck may leak through the perforations on the trays in those areas which are lowest. The vapor flow will be concentrated at the highest tray deck areas. This all results in vapor-liquid channeling, which is bad for tray vapor-liquid contacting efficiency.
The out-of-levelness of a tray that will cause a large loss in tray fractionation efficiency is related to the pressure drop of the vapor flowing through the tray deck holes or valve caps. If the low points of a tray (i.e., portions of the tray deck below the tray support ring) are less than the pressure drop of the vapor flowing through the tray deck perforations, then the liquid will leak through the tray deck and fail to come in good contact with the vapor flow.
To calculate the pressure drop of the vapor through the tray perforations, use the following equation:
DP = K x (DV ÷ DL) x V2 = inches of liquid, where DV, DL = density of vapor, liquid; V = hole velocity, fps; K = 0.3 (sieve trays); K = 0.9 (valve-cap trays); and K = 0.6 (grid-type trays).
Valve caps do not appreciably retard the tendency of liquid to leak through the tray deck panels. Bubble-cap trays would retard the liquid leakage through the tray decks, but are unfortunately seldom used in a modern distillation tower (see accompanying box).
I asked Mr. Isaac Newton while I was awaiting my transport back home from the After Life about why bubble caps were no longer used in hydrocarbon distillation service.
"Norm," Isaac explained, "the answer is that there is no answer. That goes also for the meaning of life. Only God knows."