Industry organization questions need for ULS fuels
Although ultra-low sulfur (ULS) fuels used with certain vehicle technologies will reduce vehicle CO2 emissions, there are a number of counterbalancing effects in terms of refining investment costs and plant CO2 emissions.
Potentially negative consequences in terms of air quality and greenhouse gases also require further studies.
These are the conclusions of a study by Concawe (Conservation of Clean Air & Water, Europe), Brussels, a European organization representing oil companies' interests in environmental and health protection.
Concawe defined ULS fuels as those with less than 10 ppm sulfur and evaluated the costs and benefits for these fuels for the European Union (EU).
Need for ULS fuels
The EU motor industry technology offering the most currently developed and researched route to improve gasoline engine efficiency is the direct injection gasoline (GDI) engine technology, said Concawe in its report.
The GDI engines intrinsically produce higher levels of NOx, however. The catalyst in the engines's after-treatment systems, which are sulfur sensitive, are required to reduce the final NOx emissions to desired Euro IV (2005) emission-limit levels.
Higher levels of sulfur in the fuel results in faster catalyst deactivation and more-frequent regeneration. Such regeneration increases fuel consumption and thus CO2 emissions.
Euro IV emission limits will require some diesel vehicles to be equipped with similar de-NOx devices. There are even fewer data, however, regarding sulfur sensitivity for diesel equipment.
According to the limited information published so far, de-NOx devices are operable with fuels up to 50 ppm sulfur. The motor industry is pushing a reduction in road-fuel sulfur to less than 10 ppm, however, because of the fuel-efficiency penalty suffered at higher sulfur levels.
Refinery technology
Although technologies to produce ULS fuels exist, said Concawe, implementation has a high cost and would contribute to significant additional amounts of greenhouse gas emissions from refineries.
Reducing gas oil sulfur by 3-4 orders of magnitude is largely uncharted territory. The market risks associated with breaking in new catalysts and processes by refiners are decreased reliability and localized supply disturbances.
A reduction of sulfur from 50 ppm to 10 ppm, although small in absolute terms, is not a trivial amount for refiners, said the organization. To ensure that the 10-ppm specification is met at the pump, refiners must produce fuels at a level of 6-7 ppm, that is, almost an order of magnitude less-a significant change in terms of desulfurization catalyst activity.
Because the fluid catalytic cracking (FCC) streams contribute to more than 90% of the sulfur in the gasoline pool, it must be almost completely desulfurized to meet ULS specifications. Unfortunately, desulfurization leads to olefins saturation, which reduces octane number.
Although new technologies are becoming available to remove most of the sulfur and keep octane number, some octane loss is unavoidable. High-octane additives such as reformate, oxygenates, alkylates, and butanes must be added to make up the octane.
While these components were once considered "sulfur free" because their sulfur contents were insignificant compared to the final sulfur specification, they are not considered so for ULS fuels.
At best, these additional octane components may be considered "sulfur neutral." In some cases, additional treatment would be required.
Except for hydrocracked streams that have already been desulfurized, most diesel fuels will require hydrodesulfurization to meet ULS specifications. Refiners will use more catalyst or more severe pressures to make ULS diesel.
According to Concawe, extra hydrogen and energy consumptions are relatively small because deep hydrogenation, that is, a second treatment of noble-metal catalysts, will not be required.
The main concern with diesel, the industry organization pointed out, is reliability. Before treating, diesel components can have sulfur contents up to 15,000 ppm (for example, when processing typical Middle Eastern crudes). This is largely uncharted territory for day-to-day operations. Small disturbances in the process will create off-spec product with no sink available.
Although the Swedish Class I diesel meets ULS specifications, said Concawe, it uses a much lighter than normal diesel and is produced from sweet crudes only.
Deep hydrotreating will make ULS diesels that are low in lubricity and conductivity, which will need to be compensated by an increase in additive treatments.
Logistics
Sharing of refinery oil movements, shipping systems, and distribution networks, warned Concawe, will be more difficult with ULS fuels and may create short-term supply disruptions. Refiners need to review both hardware and operating procedures.
Heating oil and jet fuels have higher sulfur contents than the ULS specifications. Contamination of ULS diesel at a rate as small as 0.1% with jet fuel could make the diesel off-spec in sulfur, although other specifications would be fine.
To ensure that the 10-ppm sulfur specification is met at the pump, refiners will have to deliver 6-7 ppm at the refinery gate. Refiners need to develop analytical methods with acceptable reproducibility and detection limits in this ppm range.
Investment, CO2 emissions
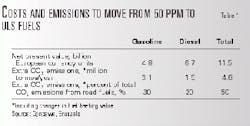
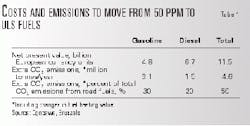
Using a linear programming model, Concawe estimated the extra cost and CO2 emissions to change from 50 ppm to 10 ppm sulfur fuels. Table 1 shows these results.
The types of investments required to achieve ULS gasoline and diesel are fundamentally different.
For gasoline, refining investment will mainly occur in plants with larger and more complex FCC units. New processing equipment, which include splitters, hydrotreaters, isomerization, and reforming units, will be related to further desulfurizing the FCC stream.
Additional energy consumption, said Concawe, is mainly a result of the energy use inherent to the additional processing plants. The additional hydrogen consumption is small.
For diesel, most refiners will invest in larger, higher-pressure hydrodesulfurization plants to meet both demand volumes and the new sulfur specifications. At the least, they will invest in major revamps of existing plants.
Generally, the new diesel-related plants will not consume more energy than the existing ones, and the extra hydrogen consumption would be small. Thus, additional CO2 emissions are relatively limited.
Investments as well as extra operating costs (for example, for extra additive-treated fuels) are high.
Air quality
Introduction of ULS fuels will have a negligible impact on either emissions or regional and urban air quality. There is a concern that warrants more study, however, and that is ULS can lead to increased ammonia emission, leading to higher levels of secondary particulates.
At a 50 ppm sulfur level, the contribution of road transport to the total SO2 emissions is extremely small. For other pollutants, said Concawe, the maximum effect is less than 0.5%.
Given the negligible impact of ULS on NOx and VOC emissions, a move to ULS fuels would contribute essentially nothing to improving ozone attainment in the EU.
Gasoline engine catalysts emit small amounts of ammonia. Once emitted in the atmosphere, ammonia forms ammonium salt, adding to the total mass of secondary particulates. Since lower sulfur in gasoline will result in higher production of ammonia, the magnitude and effects of ammonia should be studied further, suggested Concawe.
Effect on greenhouse gases
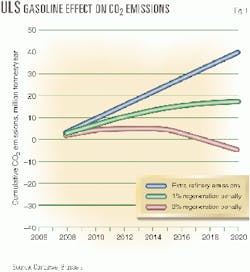
On the basis of information available about the sulfur-sensitivity of various available vehicle technologies, Concawe calculated that the CO2 increase in refineries associated with the production of ULS fuels is inadequately compensated for by the reduction in CO2 emissions associated with improved vehicle technologies.
Furthermore, N2O emissions could increase, further adding to greenhouse gases.
Concawe assumed that the fuel efficiency of gasoline engines would improve by 1-2% when reducing levels from 50 to 10 ppm. Fig. 1 shows that unless the vehicle efficiency gains are well above 1%, the CO2 increase in refineries is inadequately compensated for. For diesel engines, the development of NOx storage catalysts is even less advanced and scenarios of overall CO2 balances are less clear.
Because vehicles with a three-way catalyst produce N2O before the catalyst has reached its full operating temperature, there is a strong possibility, said Concawe, that ULS fuel production will lead to increased N2O formation.
As a greenhouse gas, N2O may be as much as 300 times more potent than CO2. Concawe estimated that a 20% increase in N2O emissions is possible with ULS fuels use. That is, an increase of 2.3 million tonnes/year CO2 equivalent in the greenhouse-gas load.