BLACK POWDER—1: Study examines sources, makeup in dry gas systems
Internal corrosion generates black powder inside pipelines, while the composition of transported gas determines the powder’s composition. Preventing its occurrence or effectively managing the effects of black powder require knowledge of its chemical composition and physical properties, formation mechanisms, and sources. Despite its common occurrence in the gas industry, black powder is not well understood in terms of composition and physical properties, source, formation, prevention, or management of its effects.
This first part of two articles reviews the recent laboratory and field work conducted at Saudi Aramco’s Research and Development Center to determine the compositions, sources, and formation mechanisms of black powder in gas transmission systems. Microhardness, nano-indentation, x-ray diffraction (XRD), x-ray fluorescence (XRF), and scanning electron microscopy (SEM) techniques, as well as several bacterial analysis methods analyzed black powder samples collected from the field.
Next week’s concluding article will describe black powder’s physical and mechanical properties and presents a summary and brief discussion of various black powder management methods.
Background
Most sales-gas pipeline operators experience black powder.1-6 It occurs in several forms, including a wet tar-like substance (Fig. 1) or a dry, fine powder (Fig. 2). Black powder occurs in both recently commissioned and older sales-gas transmission pipelines. It can contaminate the customer sales-gas supply, either interrupting the customer’s operations or reducing the quality of the customer’s products.
Black powder can also harm gas pipeline operations themselves, leading to instrument-scraping delays, reduced inline inspection accuracy, control valve erosion, and flow reduction.
Black powder additionally poses a potential health and environmental hazard, with mercury and naturally occurring radioactive materials such as Pb-210 present in some samples. Iron sulfides are also potentially pyrophoric. Such materials require special procedures for handling and disposal of any removed black powder.
Composition, sources
Black powder consists of various forms of iron sulfide, iron oxide, and iron carbonate, mechanically mixed or chemically combined with any number of contaminants such as salts, sand, liquid hydrocarbons, and metal debris. Different gas pipeline operators report different compositions for the black powder removed from their pipelines. Some literature reports black powder as being predominantly iron sulfides,1-3 while others report the complete absence of iron sulfides but the presence of iron oxides and hydroxides such as Fe3O4 and FeOOH.4 6 Still others report a combination of all of these products (iron sulfides, iron carbonates, and iron oxides).5
These products share a common source; corrosion of the internal walls of the pipeline. More specifically, reactions of iron (Fe) present in ferrous pipeline steel with condensed moisture containing oxygen (O2), hydrogen sulfide (H2S), and carbon dioxide (CO2) form black powder.
The perceived absence of condensed water in dry gas pipelines often leads to an underestimation of the internal corrosion risk.1 7 Under normal conditions, gas pipelines are under minimal corrosion risk. It is not, however, possible to eliminate water from pipelines completely.7 Water vapor can condense on the inner walls of the pipeline due to high dewpoints. It can also enter the pipeline through periodic upsets that cause moisture carryover. This water, coupled with corrosive species such as CO2, H2S, and O2, even in amounts as low as ppm levels, can result in unexpected internal corrosion and the formation of corrosion products such as FeCO3, FeS, and iron oxides, respectively.1 5
These components are benign in dry gas but become corrosive in the presence of condensed water.1
Formation mechanisms
Internal corrosion of sales-gas transmission pipelines provides the main cause for the formation of black powder. Corrosion due to H2S, CO2, and O2 in such pipelines follows established mechanisms. Simplified electrochemical reactions describing these corrosion processes and their respective corrosion products appear in the Equations box. Condensed water is a necessary condition for these reactions to proceed.
• Siderite (FeCO3) formation due to CO2 corrosion. Siderite corrosion product found in black powder forms via the chemical reaction of CO2, a naturally occurring constituent of natural gas, with condensed water, producing carbonic acid which in turn reacts directly with steel to produce FeCO3 (Equations 1-2).
• Iron sulfides formation due to H2S corrosion. Hydrogen sulfide can occur naturally as a constituent of natural gas or be produced by sulfate reducing bacteria (SRB).1 These anaerobic bacteria use the reduction of sulfate as a source of energy and oxygen (Equation 3).
Iron sulfides (FeS) corrosion products usually form from H2S dissolved in condensed moisture reacting directly with the steel wall of the pipeline (Equations 4-5).
• Iron oxides formation due to oxidation. Oxygen in gas pipelines comes from oxygen ingress through leaks at low-pressure points throughout pipeline systems.1 Oxygen ingress in gas lines can cause corrosion in small concentrations and even combustion in larger amounts.8 9 A 1988 survey of 44 natural gas transmission pipeline companies in North America showed their gas quality specifications allowed maximum O2 concentrations ranging from 0.01 mole % to 0.1 mole %, with typical values of 0.02 mole %.8 9
Oxygen content of about 0.01 mole % has little effect on steel corrosion in the presence of stagnant water inside sales-gas transmission pipelines, while 0.1 mole % produces fairly high corrosion rates. Transmission pipelines should consider limiting maximum oxygen concentrations to 10 ppm vol (0.01 mole %).8 9
In cyclical wet-dry environments with low dissolved oxygen, such as those experienced in gas pipelines, the direct oxidation of pipeline steel walls usually leads to formation of iron oxides (Equation 6).10
FeO(OH) can exist in α, β, or γ form. These type of environments lead to an unstable γ-FeO(OH), which quickly transforms to magnetite-Fe3O4 and water (Equation 7).
Water nearly saturated with dissolved oxygen will often lead to the presence of hematite (Fe2O3).
Microbiologically induced corrosion (MIC) resulting from acid-producing bacteria (APB) or iron-oxidizing bacteria (IOB) may also lead to iron-oxide formation. Again, however, condensed water is a prerequisite for these bacteria to thrive and multiply and MIC cannot occur in the absence of water.
Other sources can also produce magnetite-Fe3O4 found in black powder: mill scale, which is expected to be a minor and short-term contributor at new pipelines, and conversion, by oxidation inside the pipeline, of FeCO3, and FeS corrosion products. The conversion of FeCO3 to Fe3O4 is sluggish and occurs during dry cycles (Equation 8).11
Conversion of FeS, however, occurs rapidly and can happen during wet cycles (Equation 9).7 The produced γ-FeO(OH) will quickly transform to Fe3O4 (Equation 7).
Sampling
Analysis of sales-gas samples collected from different sampling points focused on H2S, CO2, and O2. Indirect and direct methods measured moisture content of the gas. The latter used the methanol scrubbing method and the former the panameterics dewpoint analyzer method. Collecting twice from each sampling point, once during summer and once during winter, accounted for temperature variations. No samples were collected from untreated sour gas as it is known to lead to internal corrosion of the transporting system.
Black powder sampling locations included receiving traps, pump stations, and filter cartridges. Only two of these samples were wet tar-like, with the remaining samples made up of dry, fine powder. Samples collected from manifolds and pipelines carrying associated wet sour gas had a wet slurry-like appearance. Not opening pipeline equipment until collection staff was on site avoided possible oxidation of the black powder samples.
Technicians placed the black powder samples immediately in silicon oil under inert gas (argon). XRD and XRF techniques immediately identified the phase and elemental composition of the black powder, respectively. Bacterial analysis on a number of black powder samples occurred both in-house and at the Gas Technology Institute (GTI), Des Plaines, Ill. Scanning electron microscopy (SEM) and XRD also examined the morphology and particle size of the black powder, respectively.
Hardness measurement
Two techniques measured the hardness of black powder:
- Solid-state sintering of the compacted loose powder followed by microhardness measurement of the compacted and sintered discs.
- Nano-indentation of individual black powder particles.
Hardness measurements also used conventional microhardness techniques on compacted and sintered discs. Colloidal silica polished the discs to a 20 nanometer finish. Seven measurements used a 200-g load on the cross section of the metallographically prepared discs. Chemical thermodynamic modeling and experimental trial and error ensured solid-state sintering, avoiding the formation of new phases.
Individual black powder particles underwent nano-indentation measurements. Mounting the particles in epoxy immobilized the individual grains for subsequent testing, and the samples were polished to a 20-nanometer finish using colloidal silica. A single indent occurred on individual particles, with an average of 12 particles tested per sample.
Results
Table 1 shows measured dewpoint temperatures at 130 psi by the panametrics analyzer method for gas samples collected at different sampling points. It also shows calculated moisture content at 130 psi and dewpoint temperatures at line pressures. Direct measurement of moisture content using the methanol scrubbing method showed similar moisture contents. The table shows all gas samples, except for one collected at Sample Point 6, to have a calculated moisture content exceeding the maximum moisture content of 0.112 mg/l. (7 lb water/MMcf) typically specified by sales-gas pipeline operators.
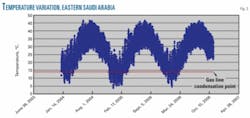
Fig. 3 shows typical temperature variations experienced in the eastern province of Saudi Arabia. Comparing Fig. 3 with Table 1 shows some dewpoint temperatures, at line pressure, are equal to or less than ambient temperatures experienced during winter. These results show high susceptibility to moisture condensation on the inner walls of pipelines during the winter.
Table 2 shows measured concentrations of gas components deemed critical to internal corrosion of sales-gas pipelines. Typical sales-gas specifications adopted by many gas line operators allow comparison. Table 2 shows O2, H2S, and CO2 levels within typical sales-gas specifications. The measured O2 level is typical of that reported by the gas industry, though measured moisture exceeds the maximum limit of 0.112 mg/l. These chemicals are benign in dry gas but form weak acids when dissolved in moisture, leading to internal corrosion of pipeline steel.
Table 3 shows XRD analysis results of black powder samples collected from sales gas and sour-gas pipelines. Black powder collected from sales-gas pipelines consisted predominantly of iron oxides with minor amounts of iron carbonate. No iron sulfides were detected in these powders. Black powder collected from sour-gas pipelines, however, consisted primarily of iron sulfides and iron carbonate.
Table 4 shows the different compounds found in black powder samples and their potential sources. Comparison of Table 4 and Table 3 shows the possibility of several potential sources for black powder in sales-gas pipelines. The moisture content values and composition of the sales gas (Tables 1 and 2, respectively) suggest internal corrosion due to low dissolved oxygen corrosion is the main source of black powder in sales-gas lines.
The conversion of primary corrosion products such as FeS and FeCO3 to secondary iron-oxide corrosion products, as well as microbiologically induced corrosion and degradation of mill scale, also make smaller contributions to the formation of black powder. H2S-induced corrosion dominates in black powder collected from sour-gas pipelines. The presence of minor amounts of Fe3O4, as well as α-and γ-FeOOH, shows oxygen ingress into these lines leading to direct corrosion and conversion of small amounts of FeS into these iron oxide species.
XRD detected elemental sulfur in the range of 3-14 wt % in some black powder samples. XRF analysis, by contrast, detected sulfur in all black powder samples. The difference suggests sulfur detected by XRF is not entirely elemental, but is combined in an amorphous compound not detectable by XRD.
The small amounts of elemental sulfur detected by XRD most likely stem from oxidation inside the pipeline of small amounts of H2S or FeS caused by oxygen ingress (Equations 10-11).
XRF analysis revealed the presence of minor contaminants such as salts, sand, metal debris (from eroded equipment), and sulfur. These minor contaminants make up 15-20 wt % of black powder. XRF also detected mercury of 0.5-2 wt % in some black powder samples.
Fourier-transformed infrared (FTIR), thermal gas analysis (TGA), and gas chromatography-mass spectroscopy (GC-MS) analyzed the wet tar-like black powder samples for organic contents. Results showed these samples containing 41 wt % organic-based liquid, with the balance being solid phase corrosion products. The liquid phase contained 36 wt % triethylene glycol (TEG), with the balance being a hydrocarbon-based liquid. The TEG desiccant likely carried over from upstream dehydration processes. TEG carryover can also contribute to internal corrosion of these pipelines.
An in-house conventional cultivation medium analyzed a limited number of the collected black powder samples for sulfate reducing bacteria (SRB), general aerobic bacteria (GAB), and nitrogen-utilizing bacteria (NUB). Analysis in the US at GTI also sought acid-producing bacteria (APB) and iron-oxidizing bacteria (IOB). SRB, IOB, and APB bacteria cause microbiologically induced corrosion (MIC) of steel in the presence of condensed moisture (water).1
Test results showed microbial corrosion to be a low-potential black powder source, caused most likely when it did occur by APB or IOB. The absence of SRB in black powder collected from sales-gas pipelines agrees with the absence of FeS in the chemical composition of black powder. The absence of SRB in black powder collected from sour gas also identifies naturally occurring H2S reactions with the steel pipeline as the source of iron sulfide (Equations 4-5).
Acknowledgment
The author acknowledges Saudi Arabian Oil Co. (Saudi Aramco) for its support and granting permission to present and publish this work.
References
- Baldwin, R.M., “Black Powder in the Gas Industry-Sources, Characteristics and Treatment,” GMRC, Report No.TA97-4, May 1998.
- Baldwin, R.M., “Here Are Procedures for Handling Persistent Black-Powder Contamination,” Oil & Gas Journal, Vol. 96, No. 43, pp. 51-58, Oct. 26, 1998.
- Baldwin, R.M., “Black Powder Control Starts Locally, Works Back to Source,” Pipeline & Gas Industry, pp. 81-87, April 1998.
- Tsochatzidis, N.A., and Maroullis, K.E., “Methods Help Remove Black Powder From Gas Pipelines,” Oil & Gas Journal, Vol. 105, No. 10, pp. 52-58, Mar. 12, 2007.
- Arrington, S., “Pipeline Debris Removal Requires Extensive Planning,” Pipeline & Gas Journal, pp. 61-62, November 2006.
- Godoy, J.M., Carvalho, F., Cordilha, A., Matta, L.E., and Godoy, M.L., “(210)Pb content in natural gas pipeline residues (“black-powder”) and its correlation with the chemical composition,” Journal of Environmental Radioactivity, pp. 101-111, 1985.
- “Corrosion Solutions for Gas Transmission Pipelines,” Honeywell Solution Note, www.honeywell.com/ps.
- Sridhar, N., Dunn, D.S., Anderko, A.M., Lencka, M.M., and Schutt, H.U., “Effects of Water and Gas Compositions on the Internal Corrosion of Gas Pipelines-Modeling and Experimental Studies,” Corrosion, Vol.57, No.3, pp. 221-235, 2001.
- Lyle, F.F., “Carbon Dioxide/Hydrogen Sulfide Corrosion Under Wet Low-Flow Gas Pipeline Conditions in the Presence of Bicarbonate, Chloride and oxygen,” PRCI Final Report PR-15-9313, 1997.
- Craig, B., “Corrosion Product Analysis- A Road Map to Corrosion in Oil and Gas Production,” Materials Performance, pp. 56-58, August 2002.
- Davis, B.R., and Calabretta, D., “Thermodynamic Analysis of Formation of Black Powder in Sales Gas Pipelines,” Saudi Aramco Report No. 6510217086, 2007.
Based on presentation to NACE Corrosion 2008 Conference, New Orleans, La., Mar. 16-20, 2008.
The author
Abdelmounam M. Sherik ([email protected]) is a science specialist at Saudi Aramco, Dhahran, Saudi Arabia. He has also served as a materials specialist at DOFASCO Inc., Hamilton, Canada, a research scientist at the National Research Council of Canada, London, Canada, and held research scientist positions at McGill and McMaster universities, Canada. He holds PhD (1994) and MS (1990) degrees in materials and metallurgical engineering from Queen’s University, Canada, and a BS (1986) in materials science and engineering from the university of Tripoli, Libya. He is a member of National Association for Corrosion Engineers (NACE).