Biocides in fracing fluid mitigate formation damage
Robert Fowles Kenneth Worsley
Weatherford International PLC
Edmonton
Clayton Smith
Weatherford International PLC
Houston
Fresh water used in hydraulic fracturing contains microbes that can proliferate in storage tanks under the right conditions.1 These microbes degrade guar-based fracing fluids, creating biofilms that can lead to formation damage and lower production. This article looks at the effects of biocides on fracing fluid to mitigate potential formation damage and address environmental concerns.
Microbial formation damage
Hydraulic fracturing fluids are mostly water-based, though oil and emulsion-based fluids are also used. The success of a fracturing program depends on its design, including using a fluid that matches the requirements of the formation.
Guar-based fracing fluids' (where a guar bean powder is added to water to form a gel) low cost and ease of handling makes them preferred, but managing fluid degradation and environmental clean-up may be difficult.2
Fresh-water microbes are a major concern when using a guar-based fluid. Among these microbes are acid-producing bacteria (APB), sulfur-reducing bacteria (SRB), and general-heterotrophic bacteria (GHB). These can be free-floating, attached to a surface, or dormant.3
A guar-based fluid without biocides can lead to biofilms that can damage a formation through bioclogging (caused by GHB), microbially-induced corrosion (caused by APB and SRB), or H2S production (caused by SRB), and reduce output.
This bioclogging may require biofilm treatment or more hydraulic fracturing to increase production, either of which raise both cost and environmental concerns about possible excessive use of chemicals.4
Biocides can be added to stored water or as part of the pad: a high-viscosity fluid containing water, polysaccharide derivatives, pH control additives, surfactants, crosslinking chemicals, breakers, and other additives.5 Treatment concentration depends on the type of biocide, the method of application, contact time available, and costs.
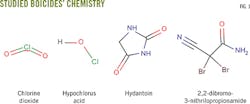
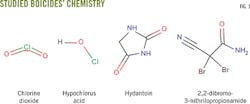
This article evaluates chlorine dioxide solutions and three biocides containing hypochlorous acid, hydantoin derivatives, and 2,2-dibromo-3-nitrilopropionamide. Testing used APB anaerobic, thioglycolate anaerobic, API SRB anaerobic, phenol red aerobic, and API aerobic microbial broth bottles (Fig. 1) to determine the biocide concentration needed to achieve a 100% and immediate kill of microbes, minimizing the risk of bacterial proliferation in a formation during fracturing. This level and rate of kill is difficult to achieve with commonly used biocides.
The article also looks at the effects of biocides on the viscosity of hydraulic-fracturing gels and slick water at elevated temperature and pressure. Biocides can affect the structure of fracturing fluids, rendering them ineffective.6
Total kill may not be achieved by some biocides because of low efficacy or the need for a higher concentration, significantly increasing costs or negatively affecting the gel or slick water.
Antimicrobial efficacy test
Water for the antimicrobial test came from a lake in Central Alberta, Canada. Efficacy tests were done in the previously mentioned bacteria broth bottles: APB anaerobic, thioglycolate anaerobic, API SRB anaerobic, phenol red aerobic, and API aerobic. Samples were tested quickly to ensure accurate results.
Researchers arranged each type of nutrient broth bottle in a dilution series of five bottles. The metal stoppers of the broth bottles were sterilized with ethanol in order to prevent contamination of the media during inoculation. Presterilized 3-ml syringes were removed from their packaging without contamination of the tips. The needle of each syringe was tightened for a closer fit to prevent air suction during operation. At least 2 ml of the water was collected into the syringe, gas bubbles removed, any remaining gas and some water displaced to 1 ml remaining, and injected into the first broth bottle.
Filling and re-injecting 3 ml of the fluid a few times without removing the syringe from the bottle vigorously mixed the broth-water. Serial dilutions then moved 1 ml of inoculated broth from Bottle 1 to Bottle 2, 2 to 3, 3 to 4 and 4 to 5, after mixing each bottle. These dilutions were the control group.7
Researchers determined the microbial demand and remaining free (residual) chlorine dioxide to be tested. The biocides (10-400 ppm) were injected in 100 ml of lake water and 1 ml of the treated water was placed into different types of broth bottles.
Serial dilutions followed the same procedures as the control group above. The broth bottles were left at room temperature and observed for 2 hrs-2 weeks. Color changes, an increasingly cloudy appearance, and the number of colony-forming units (CFU) indicated microbial growth.
The control group exhibited about 105 CFU/ml in thioglycolate anaerobic, API SRB anaerobic, phenol red aerobic, and API aerobic broths. The APB anaerobic broth showed up to 104 CFU/ml.
Chlorine dioxide (residual concentration of 10 ppm plus 27.5 ppm microbe demand, total 37.5 ppm) prevented all bacterial growth, rapidly reaching 100% kill. The biocide broths containing hypochlorous acid, hydantoin derivatives, and 2,2-dibromo-3-nitrilopropionamide did not reach 100% kill. In some cases, however, they decreased the number of CFU/ml-1 as shown in the accompanying table.
Frac gel, slick-water test
The viscosity profile of frac fluid and slick water when heated shows if these components are affected by a biocide.
To prepare a test frac gel, potassium chloride (KCl) (4g, 2% solution) is added to tap water (200 ml) in a Waring blender and mixed at 500 rpm to dissolve the KCl. The blending speed is reduced to 300 rpm and biocide, a buffer, and a gelling agent are added and mixed for 30 min. Adding a cross-linker precedes determination of vortex closure and crowning time. A rheometer measures stability of the gel (25 ml) held at at 80° C. and 200 psi for 2 hr. For a slick-water test, tap water (200 ml) is placed in a Waring blender and mixed at 300 rpm. The biocide and an anionic or cationic friction reducer (0.4 ml) are then blended for 5 min. A viscometer collects viscosity data at room temperature to 60° C. for 30 min.
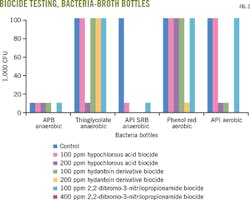
We maintained control group (biocide-free) gel integrity at 350-500 cp. With 10 ppm free chlorine dioxide, the gel was also maintained between 350-500 cp at 80° C. As the concentration of chlorine dioxide increased, however, viscosity decreased. The gel broke quickly in chlorine dioxide concentrations of more than 100 ppm (Fig 2a). An unbroken gel containing a compatible biocide is viscous with good lipping behavior. An incompatible biocide or a biocide at too high a dosage causes low viscosity and poor lipping behavior or rapid breaking of the gel.
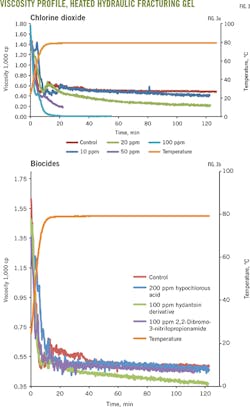
Testing of selected concentrations of the other biocides determined their effects on gel integrity. Viscosity was maintained between 350-500 cp at 80° C., using up to 200 ppm hypochlorous acid biocide and 100 ppm 2,2-dibromo-3-nitrilopropionamide and hydantoin derivative biocides. Hydantonin demonstrated the largest decrease in viscosity compared with the control group (Fig. 2b).
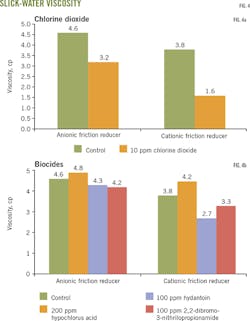
Fig. 3a-b shows the biocides' effect on selected anionic and cationic slick water. Viscosity decreased more than 30% when chlorine dioxide at 10 ppm was added to anionic slick water. A more than 50% viscosity decrease occurred when chlorine dioxide was added to cationic slick water. These results suggest that chlorine dioxide biocides will reduce the performance of some slick-water systems (Fig. 3a).
The other biocides studed had no significant effect on anionic slick water. Viscosity of cationic slick water, however, increased in the hypochlorous acid biocide. The hydantoin derivatives and 2,2-dibromo-3-nitrilopropionamide biocides decreased viscosity and therefore may affect the performance of some cationic friction reducers (Fig. 3b).
Untreated water's effect
A proppant and Barea sandstone cores were put into three conductivity cells to determine the effects of untreated water. Baseline testing used a solution of KCl (2%) a breaker, a friction reducer, and a non-emulsifier.
The cell stack was slowly ramped up to a closure stress of 2,000 psi and then heated to 112.8° C. The stack was left at temperature and pressure for 12 hr before baseline readings were taken.
About 200-250 ml of treatment fluid then flowed through each of the proppant packs at temperature and pressure. Regain-conductivity measurements were taken after flushing the packs with about 200-250 ml of 2% KCl brine.
Permeability was reduced less in source water with a biocide (11.3% of baseline) than in untreated source water with bacteria (32.2% of baseline). Permeability was not affected in KCl-only solutions.
References
1. Struchtemeyer, C.G., Morrison, M.D., Elshahed, M.S., "A critical assessment of the efficacy of biocides used during the hydraulic fracturing process in shale natural gas wells," International Biodeterioration & Biodegradation, Vol. 71, , July 2012, pp. 15-21.
2. Dawson, J.C., Cramer, D.D., Le, H.V., "Reduced Polymer Based Fracturing Fluid: Is Less Really More?" SPE Annual Technical Conference and Exhibition, Houston, Sept. 26-29, 2004.
3. Dawson, J., Wood, M., "A New Approach to Biocide Application Provides Improved Efficiency in Fracturing Fluids," SPE/EAGE European Unconventional Resources Conference and Exposition, Vienna, Mar. 20-22, 2012.
4. Ezeuko, C.C., Sen, A., Gates, I.D., "Modelling biofilm-induced formation damage and biocide treatment in subsurface geosystems," Microbial Biotechnology Vol. 6, No. 1, January 2013, pp. 53-66.
5. Fink, J., "Oil Field Chemicals," Gulf Professional Publishing, 2003, Houston, pp. 233-275.
6. Williams, D.A., Newlove , J.C., Horton, R.L., "Hydraulic Fracturing with Chlorine Dioxide clean up," US Patent 4,964,466, 1990.
7. "Field Monitoring of Bacterial Growth in Oil and Gas Systems," National Association of Corrosion Engineers (NACE) International, NACE Standard TM0194-2004, 2004.
The authors
Robert Fowles ([email protected]) has been a research and development scientist at Weatherford for 4 years. He has been project manager for different research projects involving biocides, hydrates, scale inhibitors, and heavy oil. Fowles has a PhD in chemistry from the University of the West Indies, St. Augustine, and was a postdoctoral researcher at the University of Alberta, Edmonton. He is a member of the Association of the Chemical Profession of Alberta.
Kenneth Worsley ([email protected]) is a technical service manager supporting Weatherford's R&D group in development of new products and field applications. He is involved in coordinating global projects obtaining field operating parameters used in developing lab testing to simulate production chemicals and fracturing chemicals. He holds a BS in chemistry from University of Alberta.
Clayton Smith ([email protected]) is the global director of research, development and engineering for Weatherford. He has more than 18-years' experience in the oil and gas industry and has spearheaded the development of over 500 unique chemical products spanning drilling fluids, cementing, fracturing, acid stimulation, formation remediation, and production chemicals. Clayton holds a PhD in analytical chemistry from the University of the West Indies, St. Augustine.