DNA diagnostics improve offshore reservoir analysis
Mathias Schlecht
Luke Ursell
Jordan Sawadogo
Biota Technology
Houston
DNA diagnostics provide insights for production allocation, water flooding, zonal isolation, and well integrity. Sequencing the microbial DNA in rock from producing formations results in reservoir-specific mapping from produced water or oil samples. Unlike production logs, samples can be easily obtained at any time or frequency during a well’s life cycle, and, unlike tracer logs, trace amounts of DNA can be amplified for good signal detection.
Historically, DNA diagnostics have been used in unconventional plays, but recent efforts have focused the technique on conventional plays both offshore and on land.
Background
During the last several years, operators have increasingly used DNA sequencing diagnostics to determine reservoir characteristics such as drainage rock volumes and well-to-well communication. These studies were largely based in the Permian basin where rig counts and the quality of stacked pay was high. Increasingly, operators in conventional and offshore fields are considering DNA diagnostics because of its scalability and non-invasive characteristics.
Advances in seismic, formation evaluation, and reservoir characterization tools continue to reduce uncertainties in exploration, appraisal, and development. At the same time, monitoring a field and reservoir over its life to fully understand reservoir drainage, bypassed reserves, and waterflood optimization has still not been perfected. The resulting uncertainties impact recovery rates, and the lack of temporal data results in missed opportunities for targeted workover operations and wellbore interventions.
In the era of subsea tiebacks, new methods are needed for effective and efficient production allocation from commingled wells. The effectiveness of waterfloods frequently depends on the first water break through, which limits the opportunities for optimization. Biodegraded oil renders classic tools like geochemistry ineffective for production allocation or for understanding compartmentalization. Distinguishing produced water from injected water cannot be done with conventional methods but is key to minimizing water production.
While no single tool can resolve the complexity of subsurface geology and variations in well performance, DNA diagnostics can provide insights and complement standard workflows.
This article presents conventional case studies, including one offshore field, in which DNA diagnostics were used for production allocation and water flood monitoring. Discussed applications cover the life of an asset from exploration, appraisal, and development into secondary recovery.
DNA diagnostics’ foundation
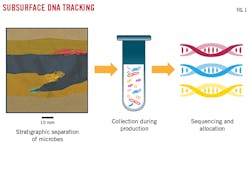
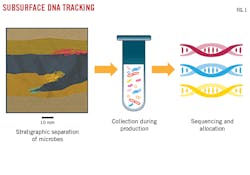
Subsurface DNA is obtained from microbes in primary and secondary pore structures, fractures, faults, and large interparticle pores. Microbes typically average 1 μm in diameter and have evolved with variations in pressure, temperature, and salinity, and the presence of assorted trace elements to better exploit and survive in their localized environment. The greater the variation between environments, the greater the differences in microbes and their DNA markers. These markers allow DNA to serve as a natural tracer that describes a given subsurface area (Fig. 1).
Sample-to-signal
DNA sequence data must be coupled with best-in-class diagnostic bioinformatic techniques, including the use of rigorous technical control samples, to identify and prevent contamination from ever-present background surface DNA. The sample types available to the oil and gas industry, specifically well cuttings, are low biomass samples, meaning that the quantity of microbial cells is several orders of magnitude less than common microbial analysis sample types, including pond water or produced fluids. The overall workflow and key considerations are shown in Table 1.
The baseline for the vertical or lateral origin of produced fluids in conventional wells can be obtained either through cores, cuttings, or downhole fluid samples. The last provide the best representative samples of subsurface fluids, but drill cuttings collected every 10 ft may be easier and less expensive to acquire.
Collection of 50-ml fluid samples occurs at the first accessible point on the surface. Sampling frequency is adjusted based on need; monthly sampling for maintenance insights or hourly sampling during active well tests. The small required volumes result in no downtime, loss of production, or risk from downhole tool or equipment deployment.
Centrifugation collects microbes from the cuttings and fluid samples. Their DNA is extracted from cells via chemical and mechanical processes, amplified, and sequenced.
After sequencing, data analysis requires bioinformatic tools that parse and simplify millions of DNA sequences into subsurface signals. Quality control steps include comparing operator samples to negative extraction controls and establishing exclusion criteria for samples that do not meet strict subsurface signal standards. This process ultimately produces a curated table containing samples by DNA markers on which all downstream analysis is performed.
Production allocation
Accurate production allocation in wells producing from multiple reservoirs is critical for actionable well surveillance, successful reservoir management, and reliable forecasting of reserves.
Production allocation can be inferred three ways: analytical methods, surface-based measurements (geochemistry), or production logging (PLT). The first is easy to compute and use but is only as robust as the data available for reservoir characterization. The second is an established technology but faces limitations in fields in which oil has been biodegraded.1 An altered composition can lower resolution to the point that it can no longer reliably unmix a commingled sample.
The third is best because it captures subsurface fluid movement, but PLT deployment cost, including production down time, resource mobilization to the rig or wellhead, and personnel time, can easily exceed $1 million in shallow water environments and $2 million in deepwater wells. Collectively, operational risks, wellbore access limitations, and operating costs may result in asset teams deploying PLTs once every 12-18 months, limiting the speed at which reservoir engineers and geologists iterate dynamic subsurface models and the quality of information available to manage the reservoir.
Offshore case study
A Gulf of Mexico operator recognized the costs and difficulties of routine PLT deployment and used DNA diagnostics as a noninvasive and cost-effective tool for routine screening of a platform’s production allocation across wells to optimize deployment timing of PLTs.
The operator deployed DNA diagnostics in a well producing from three Miocene sands in 5,000 ft of water within the stratigraphically complex Green Canyon area. The three sands were completed as a single zone, challenging reservoir drawdown management. The operator performed a PLT run on the well to compare against the DNA diagnostics. Well cuttings were collected every 10 ft through Sands A, B, and C and sent to the laboratory for DNA extraction and sequencing. A commingled fluid sample was collected and sent for DNA analysis during the run for direct comparison.
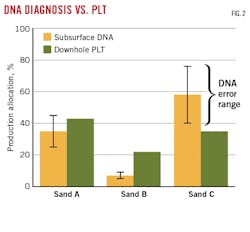
Comparing sequenced DNA data from each sand with the commingled sample allowed determination of the probabilistic strength of total fluid contribution from each sand and comparison with the PLT results (Fig. 2). Both DNA diagnostics and the downhole PLT identified Sand B as the smallest contributor to total fluid production. PLT identified Sands A and C as producing about 40% each. DNA suggested that Sand C was the larger contributor, but error bars from repeated DNA analysis showed that Sands A and C could also be producing evenly. No error bars were available for the PLT results in this case.
The case study was the first demonstrated proof of concept that noninvasive DNA-based techniques could broadly capture major findings compared with standard PLT workflows. The ease of sample collection suggests that DNA could provide weekly estimates of production allocation and increased temporal resolution for modeling. Furthermore, this monitoring can establish DNA production trends that can be analyzed over months or years and indicate when to run downhole diagnostic tools.
Waterflooding
Waterflooding is a proven secondary recovery mechanism capable of increasing recovery factors to 50% of original oil in place. At a high level, the most critical injection parameters behind maximizing a field’s recovery factor are efficient vertical and areal sweep, avoidance of early water breakthrough, and identifying the timing and rates of injection to improve oil recovery on timescales that can deliver favorable economics.
Designing appropriate injection plans depends on whether water injection is meant to 1) displace remaining oil and lower residual oil saturation or 2) maintain reservoir pressure to combat production declines from pressure depletion. Either recovery scenario is dynamic, and neither can succeed without continual monitoring, evaluation, and modification of the initial design program. Tools to help offshore operators monitor and evaluate the effectiveness of a waterflood program include soluble water tracers, streamline simulation, full-field simulation, production logging tools, and injectivity tests. Except for 4D seismic, these surveillance tools add meaningful data to a monitoring program only after injected water has moved long distances, either in the form of breakthroughs to a producer or via a change in near-wellbore water saturation.
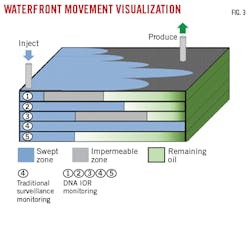
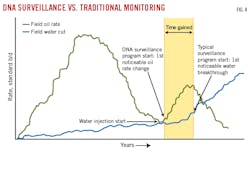
Waterflood monitoring using DNA diagnostics is better than current surveillance tools because it can both monitor changes in oil production and serve as a leading indicator for changes in fluid flow patterns through porous media (Table 2 and Fig. 3). Whereas tracers are only observed once they reach a producer, changes in DNA markers can be noted from the first detected change in oil rates.
Fig. 4 compares DNA vs. traditional surveillance start times of water breakthrough in the waterflood program of a naturally fractured reservoir. The potential time saved by switching from a lagging to leading indicator could be on the order of years for large floods. Over these timescales, DNA monitoring of the producer could identify zones susceptible to early water breakthrough, or flag intervals that have experienced early losses in injectivity, with enough advance notice to adjust and optimize injection rates and maximize flood performance. Taken to the full extent of its capabilities, DNA improved oil recovery (IOR) surveillance with real-time fluid sampling and rapid DNA sequencing can integrate surface and subsurface models for near real-time tracking of waterfront advancement and pressure maintenance injection.
Pre-offshore pilot
A pilot project on waterflooding was performed in a conventional California reservoir system as a test for offshore deployment. A total of 12 producing wells and 3 injection wells were analyzed across 2 sq miles in San Joaquin basin. The structure of the oil field is a south-eastern plunging anticline on the eastern side of San Andreas fault. Active tectonics in the area and several major unconformity events have led to structurally and stratigraphically complex reservoirs. One of the lower reservoirs remains highly compartmentalized with a complete lack of vertical transmissibility between different braided channel sands.2 Stratigraphic pinch-outs make regional horizontal permeability (kh) trends difficult to predict. These reservoir heterogeneities made the field a valuable test bed for piloting DNA IOR surveillance.
Fluid sampling took place for 3 weeks, collecting produced and injection fluids from each well on a weekly basis. Samples were collected into sterile containers under standard operating procedures to maximize subsurface microbial-community signal preservation.
The waterflooding analysis aimed to 1) determine if injected fluids contained different DNA signatures compared to produced fluids, and 2) determine the relative probability of injection fluids in each producer.
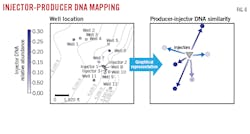
Biological similarity plots addressed the first point. The Bray Curtis distance metric, which assesses differences between each sample’s DNA markers based on abundance information, was applied to a table of DNA marker counts across the injector and producer fluid samples. Principal coordinate analysis performed on the distance matrix helped visualize sample similarity in 2D.
Each point in the biological similarity plot represents a physical fluid sample; points that are closer together in the plot represent samples with similar DNA marker profiles, while points that are further apart indicate larger DNA marker differences. Fig. 5 shows strong differences between injector and producer fluids, consistent with marked environmental differences between the samples.
Researchers next used DNA marker differences between injector and producer fluids to assess injector-to-producer contributions. Fig. 6 shows the central location of the three injectors (represented as a single icon) and six neighboring producer wells. Distance from producer to injector was not the strongest driver of DNA similarity. Results showed that the North-South orientation was strongest between injectors and producers, while two producers to the East and West had weaker DNA associations. Based on these DNA results, it appears that the strength of injector-producer connections may be driven by an underlying geological lineament running North-South, or a Northeast-Southwest meandering sand channel. Integrating these preliminary insights across larger portions of the field could identify primary paths of fluid movement between injectors and producers and optimize future infill drilling, or producer to injector conversion.
Zonal isolation, well integrity
Securing zonal isolation and maintaining well integrity throughout the life of a well is a prerequisite to accurately assessing its performance. It is also a requirement for meeting existing legal and environmental obligations for decommissioning. Zonal isolation and well integrity require continuous monitoring as either can be compromised. In early well life, micro-annuli or channeling between casing and formation during initial cement placement can compromise zonal isolation. Later, interventions such as workovers, re-completions, and fluid injection can affect isolation and integrity. At the end of well life, effective plugging and abandonment (P&A) can be compromised by large changes in pore pressure or the arrival of corrosive fluids.
Remedial costs associated with end-of-life wells are tremendous. In the UK North Sea alone, decommissioning costs are expected to exceed $21 billion over the next 10 years.3 Other regulatory regimes, such as those governing the US Gulf of Mexico, have increased the stringency of their P&A governance post-Macondo.4
Given the different circumstances across operating environments, there is a wide spectrum of deployment costs and risks among existing monitoring solutions. Few proven technologies can deliver end-to-end lifecycle monitoring without shutting in the well for direct measurement or sacrificing resolution with indirect measurement.5 DNA inflow differentiates itself from these technologies by providing actionable insights regarding remediating zonal isolation and well integrity using DNA signals directly from the formation fluids, independent of wellbore geometry, completion method, or tubing design. Three forward-looking applications of DNA inflow are illustrated in Fig. 7 and summarized in Table 3.
- Early well life. This scenario focuses on a well penetrating two formations separated by an impermeable layer. Initial production was from the deeper formation before isolating and moving uphole to perforate another payzone. Water cut increased shortly after completing the upper section, suggesting inflow from another zone based on previous production history and saturation logs. To diagnose the rise in water cut, the operator collected salinity data and ran stiff analysis.
Due to the similarity in chemical and salinity signatures of regional water sources, however, the operator was unable to identify whether water influx originated from the deeper formation or migrated from an active aquifer. Water bearing zones host more unique DNA markers than salt signatures, and DNA inflow would differentiate water sources at a resolution beyond traditional water-based geochemistry.
- Middle well life. This scenario focuses on a new drill in a mature field with depleted zones throughout the main producing intervals. Fluid losses from the cement slurry to these depleted zones create micro-annuli and loss of zonal isolation.
The pressure differential between the depleted zone and higher-pressure water-bearing zone results in crossflow through the annulus, which over time lowers productivity and increases water cut. Determining the origin of these inflows through DNA diagnostics and whether they migrated from hundreds vs. thousands of feet has profound implications on future infill drilling locations and cementing strategies.
- Late well life. This scenario focuses on legacy wells that have either been shut-in for abandonment or are no longer economically viable and facing imminent decommissioning. These wells may have produced for decades and data on past well interventions may be incomplete, complicating wellbore access. These wells must be compliant with existing regulations to fulfill legal requirements of P&A, yet monitoring zonal isolation for P&A is often complicated by the low inflow volumes relative to wellbore storage volumes.
Because DNA markers do not change as they travel from formation to wellbore, DNA inflow is uniquely positioned to analyze small volumes without the risk of signal dilution. The signal fidelity of DNA inflow in low sample-volume scenarios offers operators a cost-effective and low-risk opportunity to gain actionable insights into the current status of well integrity and tailor remediation plans by pin-pointing active inflow zones.
References
- Wilhelms, A., Larter, S.R., Head, I., Farrimond, P., di-Primio, R., Zwach, C., “Biodegradation of oil in uplifted basins prevented by deep-burial sterilization,” Nature, Vol. 411, No. 6841, June 28, 2001, pp. 1034–1037.
- Hein, Frances J., “Overview of Heavy Oil, Seeps, and Oil (Tar) Sands, California,” Heavy-oil and Oil-sand Petroleum Systems in Alberta and Beyond, Co-published by AAPG, Canadian Heavy Oil Association, and AAPG Energy Minerals Division, No. 64, January 2013, pp. 407–435.
- Adamcikova, R., “UK North Sea decommissioning: the £17 billion challenge,” Woodmac.com, Feb. 24, 2020.
- Vrålstad, T., Saasen, A., Fjær, E., Øia, T., Ytrehus, J.D., Khalifeh, M., “Plug & Abandonment of Offshore Wells: Ensuring Long-Term Well Integrity and Cost-Efficiency,” Journal of Petroleum Science and Engineering, Vol. 173, February 2019, pp. 478-491.
- “Technology Roadmap to Improve Wellbore Integrity: Summary Report,” Her Majesty the Queen in Right of Canada, as represented by Natural Resources Canada, 2019.
The authors
Mathias Schlecht ([email protected]) is the chief technology officer at Biota Technology, Houston. He has also served as vice-president enterprise technology, chief engineer, vice-president technology drilling & evaluation, and vice-president drilling services at Baker Hughes. He holds a PhD and Dipl.-Ing. (MS equivalent) in mechanical engineering from Technical University Hamburg-Harburg, Hamburg, Germany. He is a member of SPE and VDI (The Association of German Engineers).
Luke Ursell ([email protected]) is vice-president technology at Biota Technology, San Diego. He is a cofounder of Biota Technology. He holds a PhD in biochemistry and bioinformatics from the University of Colorado, Boulder.
Jordan Sawadogo ([email protected]) is a senior reservoir engineer at Biota Technology, Houston. Prior to Biota he was a reservoir engineer at Quantum Reservoir Impact (QRI Group). He holds an MSc with distinction in reservoir engineering from Imperial College, London, UK. He is an active member of SPE and is a study group treasurer within SPE’s Gulf Coast Section.