Field audits optimize Anadarko basin operator’s microbial control program
Kenneth Wunch
DuPont Industrial Biosciences
Houston
Joseph Moore
Ella Massie-Schuh
DuPont Industrial Biosciences
Wilmington, Del.
Kelly Wrighton
Michael Wilkins
Rebecca Daly
Colorado State University
Fort Collins, Colo.
DuPont Microbial Control (DMC) determined for an operator in Anadarko basin’s SCOOP and STACK plays in Oklahoma that glutaraldehyde-quaternary ammonium (glut-quat) controlled microbial contamination better than did the addition of chlorine dioxide (ClO2).
The operator questioned the effectiveness of its tandem ClO2 and glut-quat approach. Unconventional wells are susceptible to microbial contamination because high volumes of water used for hydraulic fracturing often harbor microorganisms that can colonize topside and downhole, causing corrosion and reservoir souring.
Additionally, biofilm growth within newly generated fractures can reduce overall production rates. Operators often add biocides to water and frac fluids to reduce or prevent microbial growth. Many types of chemistries are available, making it difficult for operators to decide the best formulation for specific plays.
This article describes how the authors designed a microbiological field audit using two wells. Authors outline how molecular assays and culturing techniques analyze biocide’s efficacy in the field.
An adequate biocide program quickly kills bacteria on the surface while protecting the well and the formation throughout production.
Treatment programs for fracturing fluid involve laboratory testing of a panel of biocides. Unfortunately, laboratory studies often fail to model a field’s physicochemical environment or account for additional contamination sources.
DMC audited the fracturing process to identify all contamination sources at the frac pad and measure the efficacy of each facet of the biocide treatment program. Data gathered throughout the water flow led to the following observations:
- Working tank contamination is highly variable.
- Chlorine dioxide treatment was ineffective beyond storage tanks (water residence in tanks varies from minutes to days).
- Hydration units introduced contamination regardless of whether gel was being formulated.
- Guar provided the primary contamination source from outside raw materials.
- Glut-quat treatment at the blender was effective throughout drillout.
Researchers concluded ClO2 treatment was ineffective. They suggested the operator change the biocide treatment application strategy or discontinue the ClO2 treatment.
They recommended optimizing glut-quat dosage at the blender for each frac fluid type by increasing biocide dosage for linear gel intervals and increasing glut-quat dosage by 10-30% to balance removal of ClO2.
Researchers also recommended monitoring produced waters for microbial spikes in future wells and using long-term preservative biocides to protect the reservoir
Audit design
Researchers selected two wells for the audit, seeking to control variation and similarity between them. Variables (source water quality, equipment, scheduling, weather, and hygiene practices) can alter a frac job’s day-to-day and well-to-well microbiological integrity.
An audit of one well or two nearly identical wells would be inconclusive regarding the microbial control program’s robustness or flexibility. However, selection of two wells with vastly different characteristics could convolute data analysis and distort conclusions.
Researchers sought to keep variables as similar as possible on the two target wells. They did this by:
- Selecting wells with a high risk of developing souring and corrosion issues during production. The operator said SCOOP and STACK wells with a bottom hole temperature of ≤200° F often displayed souring within the first year of production.
- Auditing both wells within 1 week to limit weather variability. The audit was done in August 2018 because warmer temperatures encourage more rapid microbial growth.
- Treating both wells with a fast-acting oxidizing biocide and an organic biocide, which enabled researchers to assess both types of microbial controls. The audit used the same biocidal active ingredients in each well although biocide product concentrations varied slightly.
Well characteristics varied in that the two wells pulled water from separate frac ponds. Different service companies handled fracing for each well. Crews used hybrid fracturing where each stage included slickwater, linear gel, and crosslinked gel.
Fracturing water’s chemical composition and contamination level changes as water flows from a frac pond to the wellhead. A versatile, optimized microbial control program reduces contamination throughout the process regardless of variabilities.
Sampling points were selected based upon where the water’s chemical makeup, contamination, or containment change.
Raw material inputs (proppant, neat-frac chemicals) represent potential contamination sources in addition to water. Researchers sampled raw materials (neat materials) from Well 1, completing a complete topside audit on Well 1, but logistics and time allowed only an abbreviated Well 2 audit. The neat form of chemicals is undiluted and straight from the container.
Water characteristics change once water is downhole, but sampling becomes more difficult. This article discusses primarily topside sampling and analysis. Sampling and data analysis of produced water continues to be studied.
Researchers used multiple complementary microbiological monitoring techniques at each sampling point such as bug bottles to collect and quantify problematic organisms, adenosine triphosphate (ATP) assay, most probable number (MPN) statistical multi-step assay to measure general heterotrophic bacteria, and quantitative polymerase chain reaction (qPCR), which rmeasures specific DNA markers to quantify microrganisms.
DMC researchers combined culture-based, metabolism-based, and molecular biology-based detection methods because no single method can capture the extent of microbiological diversity.
Results outlined
The operator reported souring in nearby wells and microbiologically influenced corrosion in production equipment. Table 1 shows the wells’ characteristics and the operator’s biocide program.
Both wells used a ClO2 generation system to rapidly decontaminate source water and added glut-quat biocide at the blender to preserve the frac fluid and decontaminate the well. The audit involved two phases: topside evaluation and well integrity. Topside sampling focused on biocides’ ability to decontaminate water quickly.
Well-integrity analysis focused on biocides’ ability to decontaminate the well and protect the reservoir in harsh subsurface conditions.
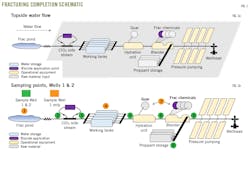
Fig. 1a shows the water flow from the frac pond to the wellhead. During normal operations, water continuously is pumped from the pond across the ClO2 sidestream, and into the working frac tanks.
Both Well 1 and Well 2 used on-site ClO2 treatment of incoming water. When water was pumped from the frac pond to the working tanks, a sidestream redirected some water for ClO2 delivery.
Due to sporadic pump rates and fracturing’s peak-water demand, working tanks serve as buffers to allow enough water to be readily available on the frac pad.
Working tanks are connected through manifolds to allow water to be pulled into the hydration unit. Researchers can take water at different rates from different tanks at any given time. Guar is injected into the hydration unit during the gel intervals of a frac stage.
During slickwater intervals, water still is passed through the hydration unit but no guar is added. All other frac chemistries are added at the blender. A conveyer adds proppant into the blender. Frac fluid then goes through a series of compressors that pressurize the fluid before it is injected into the well.
Fig. 1b shows sampling points. Nearly all samples were taken during active fracing while water was flowing, although a few samples were taken during standstill. Static samples provided insight into a microbial control program’s robustness.
Topside audit results
Sample processing occurred in the field and laboratory. Researchers compared results from the two wells and across the water flow process. To accurately compare both legacy data and future audits, each microbial quantification method was expressed in units independent of knowing the sample’s exact microbial community profile.
ATP quantification results are recorded in pg/ml (picograms per milliliter) and qPCR results are recorded in 16S copies/ml (16S is the target genetic sequence that qPCR counts to determine the number of bacteria present). Tables 2-3 show topside audit results. Table 4 shows each contamination level.
Researchers must understand variability in source water contamination to design a microbial control program. Well 1 untreated source water samples were taken during multiple days on the frac pad, and once at the frac pond about ½ mile away from the frac pad.
All measurement methods found the samples to be moderately or heavily contaminated. Researchers detected sulfate-reducing bacteria (SRB) and acid-producing bacteria (APB) via culture-based methods.
Adenosine triphosphate (ATP) and qPCR quantification methods revealed consistently high cell counts.
The degree of contamination did not change significantly daily. Well 2 source water was analyzed for 1 day with similar results to Well 1. Researchers believe contamination levels vary seasonally, with the highest growth during the summer.
The ClO2 treatment company actively monitored residual levels at the sidestream output and from lines into working tanks to ensure a stable residual level of 5-10 ppm ClO2.
Researchers did not monitor ClO2 residual levels beyond the working tanks. Water tested immediately after ClO2 treatment contained little-to-no detectable evidence of contamination. Both wells had similar results.
Well 1 used seven working tanks to hold ClO2-treated source water. Four tanks were selected for sampling and analysis. Contamination levels varied during active fracturing of each tank. Tanks 2 and 3 exhibited minimal microbial growth while water in Tanks 4 and 5 showed high DNA and ATP levels.
The MPN assay for general heterotrophic bacteria in Tanks 4-5 was >105 colony-forming units per ml (CFU/ml). Flow rates out of each tank varied. Researchers hypothesize tanks having the fastest water cycling allow the least opportunity for organisms’ regrowth.
For Well 1, each working tank has a complete water turnover every 45 min. given average frac pumping rates. Realistically, water turnover is more variable than this average.
Water can be pulled from single tanks, which accelerates turnover, or operations can be stopped, which slows turnover. During Well 1’s audit, crews delayed fracturing for multiple days, during which time the water in all tanks grew stagnant.
To test a hypothesis and discern the effect of paused frac operations on tank water, researchers used 16S ribosomal RNA iTag amplicon sequencing to profile the microbial community membership and abundance.
The 16 S rRNA is present in all bacteria and used as a genetic marker to identify and track particular types of bacteria.
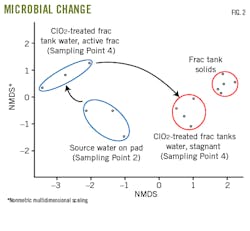
Samples included source water on the frac pad (Sampling Point 2), frac tank water during active fracturing, stagnant water (Sampling Point 4), and scrapings of solid material from the fracturing tank walls (Fig. 1b).
Solids are scrapings from the side and bottom of the tanks, resulting in biofilm and other solid material.
Researchers used amplicon data to construct a nonmetric multidimensional scaling (NMDS) ordination where dots indicate samples. Fig. 2 shows highly similar microbial communities are close together on the ordination while dissimilar communities are farther apart.
Microbial communities proved statistically different by sampling points except for the tank water. Water chemistry differed if samples were taken during active fracturing or after the tank water had stagnated for 4 days.
Fig. 2 shows stagnant-tank water microbial communities are most like the frac-tank solids, suggesting ClO2-treated water becomes more contaminated as it stagnates.
Sampling Points compared
Regarding Sampling Points 5 and 6, water samples taken from the hydration unit (where flow from multiple storage tanks coalesced) provided a more accurate frac water contamination profile than water samples from isolated tanks or directly after ClO2 treatment.
On both Wells 1 and 2, this sample showed ATP and qPCR contamination.
Data suggests that, at any given time, the water chosen for frac fluid is only as clean as the dirtiest working tank. Researchers note the anaerobic culture-based enumeration for coalesced post-tank water in Well 2 gave significantly lower counts than did ATP- and DNA-based enumeration.
Well 2 had fewer activity suspensions so its environment likely stayed more aerobic and did not allow for as much anaerobic organism regrowth. Observations corroborated that the ClO2 treatment’s effectiveness depends on environment and upon residence time to key process points
Substantial organismal regrowth was observed before entering the hydration unit at both wells. Researchers concluded that regardless of gel or slickwater, ClO2 treatment is ineffective at preserving fluid beyond the working tanks and fails to control microbes in downhole frac fluid.
At both wells, hydrated gel samples were taken after the hydration unit. These samples were highly contaminated, exhibiting SRB of levels equal to or greater than the initial source water.
The high organism counts likely were caused at least partially from addition of guar, which can be an energy source for microbes.
A suite of chemicals and proppants (Sampling Points 7 and 8) are added to frac fluid to tune physicochemical properties for increased frac efficiency. The main additives are proppant, friction reducer, and guar.
These additives were sampled in their neat form to determine if they harbored microorganisms that might contribute to frac fluid contamination. Two types of proppant were used on both wells: 40–70 mesh and 100 mesh sands. Samples were taken at the conveyer taking sand into the blender. The proppants were vortexed in phosphate-buffered saline to dislodge any organisms from the sand, and the resulting liquid was analyzed for ATP and qPCR.
Both methods for all samples returned very low results, suggesting that the proppants were not a significant contamination source. Friction reducer and guar samples were taken from Well 1’s pump lines.
The friction-reducer sample showed minimal evidence of bacterial contamination, and the guar sample harbored a moderate level (102 CFU/ml) of general heterotrophic bacteria. Researchers expect these organisms could proliferate when the neat guar is fully hydrated.
Biocide application
Most frac fluid additives (including biocide) are applied at the blender (Sampling Point 9).
Researchers took samples from each well during a single stage, allowing a relatively controlled comparison of the organic biocide package efficacy across slickwater, linear gel, and crosslinked gel frac fluids.
Table 2 shows the operator used a consistent biocide dosage in all fluid types. Frac fluid in Well 1 was dosed with 62.5 ppm glut and 15 ppm quat, while frac fluid in Well 2 was dosed with 62.5 ppm glut and 30 ppm quat.
These concentrations are in terms of active ingredient. Biocide products often are diluted, and the appropriate conversion of biocide product dosage (expressed in gallons per thousand or l./cu m) to pure active ingredient should be made before comparing other operations to this audit.
Organic biocides take effect slower than oxidizers so as to accurately compare the efficacy of the two biocide dosage points. Biocide samples added at the blender were allowed to contact the samples for 1 hr before researchers analyzed them.
On Well 1, samples were almost entirely decontaminated after 1 hr with only a single aerobic APB bug bottle among the slickwater and linear gel samples. No bug bottles showed contamination in the crosslinked gel sample.
Other field analyses could not be performed on these frac fluid samples due to the fluid’s complexity. More detailed and complete laboratory studies were performed on samples sent to the lab.
Glut-quat addition at the blender not only cleans residual contamination unaddressed by the ClO2 program but also decontaminates the well and protects the reservoir.
It’s extremely difficult to measure biocide efficacy downhole, so this audit attempted to measure well decontamination in two ways. First, frac fluids from the field (Sampling Point 9) were incubated in the laboratory and evaluated over time.
Second, samples were taken during well drillout to measure contamination of actual downhole fluids exposed to real-world well conditions.
In addition to analyzing Well 1 samples at 1 hr of biocide-fluid contact (to determine the biocides’ short-term efficacy to remove residual topside contamination), frac fluid samples were analyzed after 2 weeks of biocide-fluid contact at room temperature to provide laboratory-based data on the biocides’ ability to decontaminate the well longer-term (Table 5).
Researchers collected only limited samples from Well 2 because of safety considerations around the frac pad. The Well 2 samples were submitted for analysis 2 weeks later.
The 2-week period gauged the biocide’s ability to protect the fluid over longer periods after reaching the reservoir. Although this measurement is not a substitute for field data, it allows insight into the degree of preservation of each frac-fluid type.
Details on each frac stage were unavailable once the fluids mixed downhole. Well 1 and Well 2 samples showed little difference in the degree of fluids preservation.
While all fluid types remained well preserved in Well 1, the linear gel used in Well 2 exhibited significant microbial growth after 2 weeks, despite Well 2 having a slightly higher organic biocide dosage. This suggests that while the slickwater and crosslinked gel fluids are effectively preserved, the linear gel should be dosed with a higher biocide concentration.
Researchers suspect that linear gels are more easily metabolized by organisms. Well 2’s linear gels might be susceptible to growth for various reasons. For example, the gel might have been contaminated initially with more organisms.
Field Analysis
After fracing, the operator used coiled tubing to drill out plugs at each stage. Flowback from the drillout includes water from the wellbore, providing the first examination of biocide efficacy downhole.
Researchers took samples only at Well 1 during drillout. Crews took fluid samples directly from the coil tubing flowback line before the fluid entered topside containment. Two unique stages were sampled, including the same stage audited during the bulk of topside sampling.
Control measurements also were taken from the working tank that supplied the fluid used to power the drill (Table 6).
Corroborating laboratory results, DMC found drillout flowback from two independent stages were free from detectable levels of contamination and exhibited very low DNA reads by qPCR.
Flowback water was cleaner than the input water used to power the coiled tubing drill bit. These results confirm that the glut-quat biocide treatment applied at the blender cleaned up residual contamination not addressed by ClO2 treatment and protected fluids in the lateral after frac until production.
Both wells showed microbe growth at similar points in the fracturing process. DMC gained unprecedented insight into the efficacy of two common biocide treatment strategies. Microbial growth was observed after biocide addition. ClO2 lost control at the working tanks, but glut-quat kept control of growth in the wells and reservoir until drillout.
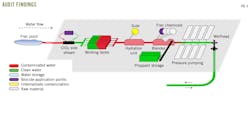
Fig. 3 summarizes the audit findings. Some working tanks were contaminated, leading to contamination of coalesced water. Biocide treatment at the blender preserved fluids through drillout.
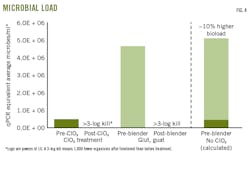
Fig. 4 compares microorganism load between fluids directly before the treatment points. Researchers added source water and hydration-unit water to create the anticipated bioload of a fluid not exposed to ClO2 treatment. Values were averaged from all relevant qPCR readings at Wells 1 and 2.
DMC recommended removing the ClO2. The operator’s primary concern was that removal would increase the bioload for the glut-quat treatment at the blender. The glut-quat would have to kill organisms both from the frac pond and at the working tanks and hydration unit.
Researchers calculated the average bioload of the source water before ClO2 treatment and compared it to the average bioload of water entering the blender before glut-quat treatment. To calculate a microbial equivalent, researchers divided the 16S copy number by the standard value of 3.6 average 16S copies/cell.
Presumably, removal of ClO2 treatment would result in the organic biocide encountering additional contaminant cells from the frac pond.
Researchers calculated a hypothetical cell density that the glut-quat treatment would encounter without the ClO2 treatment supplement. Fig. 4 shows removal of ClO2 would likely result in an average ~10% increase in bioload at the blender.
Consequently, DMC recommended an overall dosage increase of 10-30% glut-quat (depending on input water contamination level) to compensate for ClO2 removal.
Bibliography
Bottero, S., Picioreanu, C., Enzien, M., Van Loosdrecht, M., Bruining, J., and Heimovaara, T., “Formation Damage and Impact on Gas Flow Caused by Biofilms Growing Within Proppant Packing Used in Hydraulic Fracturing,” Society of Petroleum Engineers International Symposium and Exhibition on Formation Damage Control, Lafayette, La., Feb. 10-12, 2010.
Caporaso, J., Lauber, C., Walters, W., Berg-Lyons, D., Huntley, J., Fierer, N., Owens, S., Betley J., Fraser, L., Bauer, M., Gormley, N., Gilbert, J., Smith G., and Knight, R., “Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms,” ISME Multidisciplinary Journal of Microbial Ecology, Vol. 6, 2012, pp. 1621-2624.
Corrin, E., Rodriguez, C., and Williams, T., “A Case Study Evaluating a Co-Injection Biocide Treatment of Hydraulic Fracturing Fluids Utilized in Oil and Gas Production,” National Association of Corrosion Engineers (NACE) International Corrosion Conference and Expo, Dallas, Mar. 15-19, 2015.
Crolet, J. "Microbial Corrosion in the Oil Industry: A corrosionist’s view,” In Petroleum Microbiology, Chapter 8, ASM Press, Washington, DC, 2005.
Nadkarni A., Martin E., Jacques, N., and Hunter, N., “Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set,” Microbiology, Vol. 148 (Part 1), January 2002, pp. 257-266.
Paulus, W., “Directory of Microbicides for the Protection of Materials: A Handbook,” Springer Publishing, New York City, 2005.
Voordouw, G. “Production-related petroleum microbiology: progress and prospects,” Current Opinion in Microbiology, Vol. 22, No. 3, pp. 401-405.
Youssef, N., Elshahed M., and McInerney M., “Microbial processes in oil fields: culprits, problems, and opportunities,” Advanced in Applied Microbiology, February 2009. pp. 141-251.
The authors
Ken Wunch ([email protected]) develops DuPont’s strategy for microbial control for oil and gas applications worldwide. He is the technical leader of the DMC team responsible for developing new products and lead technical partner for marketing and the customer application centers. Wunch received his PhD in environmental microbiology (1996) from Tulane University in New Orleans followed by post-doc research on hydrocarbon bioremediation of the Valdez oil spill. Subsequently, he accepted an assistant professorship at the Texas Research Institute for Bioenvironmental Studies and became director of the Sam Houston State University Disease Vector Program for the Air Force Border Health and Environmental Threats Initiative. Wunch moved into petroleum microbiology at Baker Hughes with responsibilities in production chemistry, development and application of oil and gas microbiology technologies, microbially influenced corrosion (MIC), sulfide abatement, and corrosion inhibition. He joined BP PLC as a production microbiologist responsible for development and implementation of research and development strategies for reservoir souring and MIC before his current role at DuPont.
Joseph Moore ([email protected]) is an applications specialist at DMC, where he focuses on technical service and development for biocide applications in the oil and gas industry. Moore troubleshoots oilfield microbial contamination events and develops optimized biocide solutions to control problematic microbes for customers. He has written articles for multiple academic and industrial journals and has presented at chemistry, bacteriology, and industry conferences across North America. Moore earned a PhD (2015) in chemistry from the University of Wisconsin–Madison where he designed and synthesized chemical tools to influence bacterial communication.
Ella Massie-Schuh ([email protected]) is a senior applications technologist working with the DuPont Microbial Control business applications and technical service group. She performs field audits in North America, evaluating biocide efficacy, and performing various molecular analyses. Massie-Schuh joined the microbial control team in 2013 after earning a BS in microbiology from Ohio Wesleyan University (2011), and an MS (2013) in microbiology and immunology from Temple University School of Medicine.
Kelly Wrighton ([email protected]) is an assistant professor for Colorado State University’s department of soil and crop sciences. Her research applies tools to ecosystems, ranging from shales to the human gut, to understand microbial community function. She previously worked as an assistant professor at Ohio State University. She received a PhD (2010) from the University of California at Berkeley, and a MS (2005) and BS (2001) from California Polytechnic State University (San Luis Obispo).
Michael Wilkins ([email protected]) is an assistant professor for Colorado State University’s department of soil and crop sciences. His research involves metabolism and physiology of subsurface microbial populations, focusing particularly on unconventional hydrocarbon reservoirs. He previously worked as an assistant professor at Ohio State University and as a senior staff scientist at Pacific Northwest National Laboratory. He earned a PhD. (2006) in geomicrobiology at the University of Manchester (UK) and a BS (2002) in microbiology at the University of Birmingham (UK).
Rebecca Daly ([email protected]) is a Colorado State University research associate. She studies the microbiology of hydraulically fractured wells with a focus on viruses infecting subsurface bacteria and archaea. She joined Kelly Wrighton at Colorado State University in 2018 after having worked with Wrighton at Ohio State University, starting in 2013. She earned a BA (2005) in biology from Colorado College.