Ionic liquid alkylation process produces high-quality gasoline
A new composite ionic liquid (IL) alkylation process has been proven in a pilot plant and retrofitted into an existing 65,000-tonne/year H2SO4 alkylation unit in China. This article discusses the new process, ionikylation, and presents results from the pilot plant and retrofitted unit.
Process reactions take place at ambient temperatures and moderate pressures. Alkylate from the ionikylation process compares favorably to alkylate from HF and H2SO4 units but without the safety and environmental concerns.
Alkylation
Isobutane alkylation is a common refinery process used to produce high-quality gasoline (OGJ, Nov. 12, 1990, p. 79). The most desirable components in alkylate gasoline are trimethylpentanes (TMPs), which have research octane numbers (RONs) greater than 100.
Conventional alkylation processes use either H2SO4 or anhydrous HF acid as catalysts. This has significant safety and environmental concerns due to the handling of large quantities of spent H2SO4 or hazardous HF.
Solid acids have shown promise as less hazardous catalysts for alkylation1 and have been subjected to extensive pilot scale testing.2 3 Solid-acid catalysts, however, deactivate rapidly, resulting in low product yield and loss of reaction selectivity.4
The rapid deactivation is due to a buildup of carbenium ions on active sites of the solid catalysts. Once this carbonaceous material is formed, it is difficult to remove from the narrow catalyst pores. Moreover, the cost of solid-acid catalysts is relatively high and there is no technically sound method for regenerating spent solid-acid catalysts.
Ionikylation
Ionikylation is an environmentally friendly and energy efficient isobutane alkylation process. The process uses a composite-IL as homogeneous catalyst for alkylation reactions at ambient temperatures and moderate pressures.
ILs are ionic, salt-like materials that are liquid at less than 100° C.5 ILs have been historically used as solvents and homogeneous catalysts6 due to their negligible vapor pressure, good solubility to a wide range of organic and inorganic compounds, and ability to be recycled for reuse.7
Acidic chloroaluminate (III) IL has been used as a homogeneous catalyst for isobutane alkylation. Its use eliminates the diffusion limitation present with solid-acid catalyst systems, and alkylated gasoline is easily separated from the ionic liquid.8-10
TMP yield and selectivity, however, are low in systems with conventional-IL-with and without adjusting the IL’s acidity by varying either the molar fraction of aluminum chloride (AlCl3) of the IL or adding hydrochloric acid (HCl).10-12
A study showed that adding aromatic hydrocarbons and metal chlorides to aluminum chloride-dialkyl ether complex enhanced TMP selectivity. It is theorized that adding aromatic hydrocarbons and metal chlorides enhances the acidity of catalyst for alkylation reactions and inhibits undesirable side reactions such as isomerization and cracking.13
In subsequent experiments, aromatic hydrocarbons and cuprous chloride (CuCl) were added to a conventional-IL, which showed high TMP yield and selectivity.10 11 14 Adding CuCl to an aluminum chloride-dialkyl ether complex or conventional-IL, however, results in formation of a fine suspension that is difficult to separate and recycle in a continuous-flow system.
Composite-IL catalyst
A further development in the commercialization of IL-catalyzed isobutene alkylation is the development of a composite-IL catalyst. It is a liquid compound, which is synthesized with a conventional-IL catalyst and CuCl.15 The composite-IL catalyst has anions in the form of ligands with two or more metallic centers.
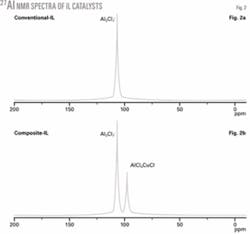
Fast-atom bombardment mass spectrometry (Fig. 1) and 27Al nuclear magnetic resonance (NMR, Fig. 2) show that a relatively high quantity of multi-center ligands of AlCl4CuCl- are formed in the composite-IL catalyst. By comparison, only a few AlCl4CuCl- ligands were detected in the IL-CuCl system when CuCl was added to a conventional-IL catalyst.16
null
Pilot plant
Extensive bench-scale laboratory tests were conducted to optimize the composite-IL catalyst’s performance in alkylating isobutane.10 The ionikylation process was demonstrated in a continuous-flow pilot unit with 4-l./hr equivalent alkylate gasoline production for 60 days.
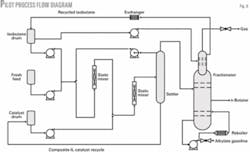
Fig. 3 shows the process flow diagram of the pilot unit. The pilot unit was constructed from carbon steel.
The composite-IL catalyst used in the pilot test unit was prepared commercially with industrial-grade chemicals. This was done to determine the effect of impurities on the composite-IL catalyst due to isobutane alkylation reactions. The composite-IL catalyst was also subjected to an aging test by storing it in a tank for 8 months before the pilot test.
Fresh feed was a mixture of isobutane and a heavy C4 fraction (primarily butene-2) in a 1:1 ratio. These streams were obtained from a commercial refinery. Fresh feed, recycled isobutane, and a mixture of excess isobutane and alkylate gasoline from the top of the settler were fed to the first static mixer.
The stream from the first static mixer was combined with the recycled composite-IL catalyst and fed to the second static mixer where the isobutane alkylation reactions occurred at 15° C. and 0.4 MPa.
Reaction products then flowed to a settler. The composite-IL catalyst, which is heavier than alkylate gasoline, was collected from the bottom of the settler and recycled and reused. A split stream of excess isobutane and alkylate gasoline at the top of the settler were recycled to the first static mixer. Remaining products from the top of settler were fed to a fractionation column.
Isobutane at the top of the fractionation column was cooled and recycled to the first static mixer. It maintained a relatively high isobutane-to-olefin (I:O) ratio and low reaction temperature. Product n-butane and alkylate gasoline were obtained from the middle and bottom of fractionation column, respectively.
The overall I:O ratio of reactants in the reactor was 500, which was controlled with the flow rate of recycled isobutane and the mixture of excess isobutane and alkylate gasoline. The reaction time was 10 min, which depended upon the residence time in the second static mixer and sedimentation time in settler.
The volumetric ratio of composite-IL catalyst to the stream from the first static mixer was 1.2. This was done to maintain the composite-IL catalyst as a continuous liquid phase, which helped achieve high TMP selectivity.
Ionikylation performance
Olefin conversion was constantly more than 99% for the entire pilot test period.
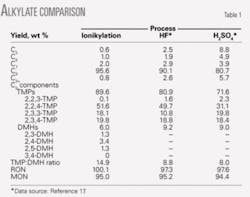
Table 1 summarizes the product yield and properties of alkylate gasoline. The C8 yield in alkylate gasoline was higher than 95 wt % and the yield of TMPs was 90%.
The ratio of TMPs to dimethylhexanes (DMHs) in alkylate gasoline is an important key criterion for comparing the performance of alkylation catalysts. Alkylate from the composite-IL catalyst had a high TMP:DMH ratio, indicating that a relatively small amount of undesirable side reactions such as isomerization and cracking occurred. The high C8 yield and TMP selectivity of composite-IL catalyst are due to high concentration of AlCl4CuCl- ligands.
Performance of the ionikylation process in the continuous pilot test was comparable to that of the bench-scale test. This shows that the commercial composite-IL catalyst performed in a similar manner as that developed in the laboratory, and its activity was stable.
Neither the composite-IL catalyst nor its decomposed species was detected in alkylate gasoline, indicating good separation of composite-IL catalyst from alkylate gasoline and chemical stability of composite-IL catalyst. Metallurgical inspection of the pilot unit indicated that no detectable corrosion occurred in the process units during the 60-day continuous pilot test.
Also included in Table 1 are performance data of H2SO4 and HF catalyzed isobutane alkylation processes.17 In general, the ionikylation process produces a higher-quality alkylate gasoline than either H2SO4 or HF alkylation processes.
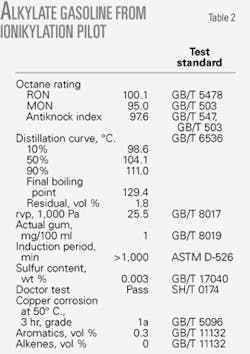
Table 2 lists the detailed properties of alkylate gasoline produced by the ionikylation process. The quality of product from the ionikylation process exceeded the specifications for gasoline, such as higher RON and MON, lower rvp, and temperature at 50% and 90% distillation yield.
Benefits
Operating conditions in the ionikylation process are similar to those of commercial H2SO4 and HF alkylation processes. The reactor used in ionikylation, however, is a commercial static mixer that is much simpler and cheaper than the reactor used in the H2SO4 alkylation process.
Because ionikylation uses a composite-IL catalyst that is noncorrosive, carbon steel can be used for the hardware such as reactors, piping, tanks, pumps, and valves. The control corrosion test of the composite-IL catalyst on carbon steel indicated that the corrosion rate was less than 0.001 mm/year.
Ionikylation can be easily retrofitted to an existing H2SO4 or HF alkylation unit.
Commercialization
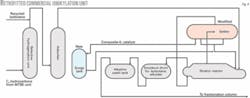
The successful demonstration of the ionikylation process prompted PetroChina to retrofit an existing 65,000-tonne/year (tpy) H2SO4 alkylation unit (Fig. 4). A new surge tank was added to recycle the composite-IL catalyst and allow the spent catalyst to settle.
The internals of the settler were modified to enhance the separation of composite-IL catalyst from alkylate gasoline. The operating conditions in the selective hydrogenation unit were modified to meet the required concentration of 2-butene in the feed C4 fraction for ionikylation.
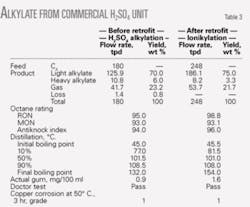
Table 3 shows commercial performance data before and after the retrofit. The yield and RON of commercial ionikylation alkylate gasoline are 75 wt % (based on the amount of C4 fraction from the MTBE unit upstream) and 98.8, respectively. This is higher than the yield and RON from H2SO4 alkylation (73 wt % yield and 95 RON) before the retrofit.
The retrofit also increased the process unit’s capacity by 40%, to 248 tonnes/day (tpd) from 180 tpd. The economics of increased yield and RON of alkylate gasoline from ionikylation are attractive, even though the cost of the composite-IL catalyst is more than H2SO4.
Compared with the pilot test results, the commercial performance of retrofitted ionikylation was less than optimal. This is due to the use of an existing Stratco reactor system, which has a long reaction time.
This results in increased side reactions such as cracking and isomerization, which form light and heavy components in alkylate gasoline. These are evident from the shift in distillation temperature at 10% yield (81.5° C. vs. 98.6° C.) and final boiling point (154° C. vs. 129.4° C.) when comparing the pilot results with those of the retrofitted unit.
We are currently conducting process optimization work to improve the commercial performance of ionikylation further by varying the size and type of static mixer and potentially replacing the Stratco reactor with a static mixer to minimize reaction time.
Acknowledgments
The National Natural Science Foundation of China provided strategic research grants and PetroChina provided financial support, feedstocks for the pilot test, and technical consultation for commercial implementation.
References
- Corma, A., and Martinez, A., “Catalysts and processes for isoparaffin-olefin alkylation,” Catal. Rev., Vol. 35 (1993), No. 4, pp. 483-570.
- Hommeltoft, S.I., Ekelund ,O., and Zavilla, J., “Role of ester intermediates in isobutene alkylation and its consequence for the choice of catalyst system,” Ind. Eng. Chem. Res., Vol. 36 (1997), No. 9, pp. 3491-97.
- Pryor, P.S., “Approaches to alkylation: a world review,” Petroleum Technology Quarterly, Winter 2004, pp. 69-76.
- Weitkamp, J., and Traa, Y., “Isobutane/butene Alkylation on Solid Catalysts,” Catal. Today, Vol. 49 (1999), No. 2, pp. 193-99.
- Rogers, R.D., and Seddon, K.R., “Ionic liquids: Solvents of the future?,” Science, 2003, Vol. 302 (2003), No. 5646, pp. 792-93.
- Rogers, R.D., and Seddon, K.R., eds., “Ionic Liquids as Green Solvents: Progress and Prospects,” ACS Symposium Series 856, American Chemical Society, Washington, DC, 2003.
- Welton, T., “Room-temperature ionic liquids. Solvents for synthesis and catalysis,” Chem. Rev., Vol. 99 (1999), No. 8, pp. 2071-83.
- Chauvin, Y., Hirschauer, A., and Oliver, H., “Alkylation of isobutane with 2-butene using 1-butyl-3-methylimidazolium chloride-aluminium chloride molten salts as catalysts,” J. Mol. Catal., Vol. 92 (1994), No. 1, pp. 155-65.
- Yoo, K., Namboodiri, V.V., Varma, R.S., and Smirniotis, P.G., “Ionic liquid-catalyzed alkylaton of isobutane with 2-butene,” J. Catal., Vol. 222 (2004), No. 2, pp. 511-19.
- Huang, C.P., Liu, Z.C., Xu, C.M., Chen, B.H., and Liu, Y.F., “Effects of additives on the properties of chloroaluminate ionic liquids catalyst for alkylation of isobutane and butene,” Appl. Catal., A: General, 2004, No. 277, pp. 41-43.
- Olivier-Bourbigou, H., and Hugues, F., “Ionic Liquids in Synthesis,” Wiley-VCH, Weinheim, Germany, 2003, pp. 258-81.
- Smith, G.P., Dworkin, A.S., Pagni, M.R., and Zingg, S.P., “Brönsted superacidity of HCl in a liquid chloroaluminate, AlCl3-1-ethyl-3-methyl-1H-imidazolium chloride,” J. Am. Chem. Soc., Vol. 111 (1989), No. 2, pp. 525-30.
- Roebuck, A.K., and Evering, B.L., “Isobutane-olefin alkylation with inhibited aluminum chloride catalysts,” Ind. Eng. Chem. Prod. Res. Dev., Vol. 9 (1970), No. 1, pp. 76-82.
- Chauvin, Y., Hirschauer, A., and Oliver, H., European Patent 0709356A1, 1996.
- Liu, Z.C., Huang, C.P., and Xu, C.M., Chinese Patent 02149296.4, 2004.
- Liu, Z.C., Zhang, Y.H., Huang, C.P., Gao, J.S., and Xu, C.M., “Effect of CuCl additive on catalytic performance of Et3NHCl/AlCl3 ionic liquid in C4 alkylation,” Chinese J. Catal., Vol. 25 (2004), No. 9, pp. 693-96.
- Ma, B.W., ed., “Clean Fuel Production,” China Petrochemical Press, 2001, pp. 112.
The authors
Zhichang Liu is deputy director of the State Key Laboratory of Heavy Oil Processing, associate dean of the Faculty of Chemical Science and Engineering, and an associate professor at the China University of Petroleum, Beijing. He specializes in clean fuels and ionic liquids processes. Liu holds a PhD in chemical engineering from Shanxi Institute of Coal Chemistry, Chinese Academy of Sciences.
Rui Zhang is PhD candidate at the China University of Petroleum. His area of study is isobutane alkylation using ionic liquids.
Chunming Xu is director of the State Key Laboratory of Heavy Oil Processing and vice-president of academics at China University of Petroleum. His research interests include petroleum processing, FCC kinetics of heavy oil, numerical simulation of FCC riser reactor, heavy oil characterization, and ionic-liquid catalyzed alkylation. Xu holds a PhD from the China University of Petroleum.
Rongan Xia is vice-president of PetroChina Lanzhou Petrochemical Co. He has more than 20 years of operations and management experience. Xia holds an MBA from Dalian University of Technology and is currently a PhD candidate at the China University of Petroleum.