How temp. affects H2O solubility in cycloalkanes
Carl L. Yaws
Urvashi Yadav
Lamar University
Beaumont, Tex.
The solubility of water in cycloalkanes (cyclopentanes and cyclohexanes) found in crude oil as a function of temperature has been calculated.
In addition, we have developed a new correlation for solubility of water in crude oil that provides reliable solubility values down to very low concentrations (ppm). The correlation is based on boiling point temperature of the cycloalkane. Correlation values and experimental data are in agreement.
The results are usable in engineering applications involving processing, safety, hazard, environmental, and environmental considerations.
Previous articles (OGJ, Mar. 2, 2009, p. 52; Feb. 1, 2010, p. 54) provided data for water solubility in hydrocarbons at ambient temperature. Another (OGJ, Dec. 6, 2010, p. 130) provided data for the effect of temperature on the solubility of water in alkanes.
The present article provides data for the effect of temperature on the solubility of water in cyclyoalkanes. This article and the immediately previous one cover prominent alkanes and cycloalkanes contained in crude oil.
In refinery operations, many different temperatures are encountered in the processing of hydrocarbons in crude oil. This and the previous article provide data for the effects of temperature that are especially applicable for many different temperatures encountered in the processing of such hydrocarbons as alkanes and cycloalkanes in a refinery.
Water solubility
The importance of the solubility of water in hydrocarbons such as cyclyoalkanes in crude oil will increase in view of processing, safety, hazard, environmental, and environmental considerations focusing on product quality and equipment sustainability. The following brief discussion illustrates the importance.
Any processing that lowers temperature to near the freezing point of water may result in the formation of solids (freezing of water or hydrate formation). Such formation will affect both fluid flows in piping and operational characteristics of equipment. For catalytic reactions, any water in the hydrocarbon may poison the catalyst that promotes the hydrocarbon reaction.
For reactions in general, any water in the reaction species may result in the formation of undesirable by-products issuing from the hydrocarbon reaction. The presence of water in the product may degrade quality and, if sufficient water is in the product, it may prove to be unusable by the customer.
This brief discussion indicates that solubility of water in hydrocarbons contained in crude oil is important in engineering applications involving processing, safety, hazard, environmental, and environmental considerations.
Correlation
The correlation for water solubility as a function of temperature is based on Equation 1 in the accompanying equations box. The correlation applies to a range of about 298 to 478 K.
The coefficients (A, B, and C) for the correlation were determined from regression of the available data. In preparing the correlation, we conducted a literature search to identify data source publications.1-19
We screened the publications and copied appropriate data. These data were then keyed into the computer to provide a data base for which experimental data are available. The database also served as a basis to check the accuracy of the correlation.
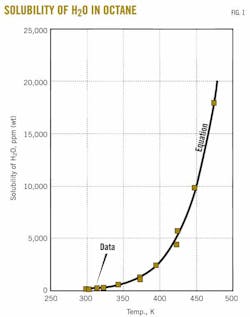
Fig. 1 shows the solubility of water as a function of temperature for a representative cycloalkane (cyclohexane). For the graph, we selected the data of Englin et al.,2 Marche et al.,3 and Tsonopoulos and Wilson.12 Fig. 1 discloses favorable agreement of data and equation.
Displaying 1/3 Page1, 2, 3Next>
View Article as Single page