How temp. affects H2O solubility in cycloalkanes
View Article as Single page
Tabulation, estimation of equation
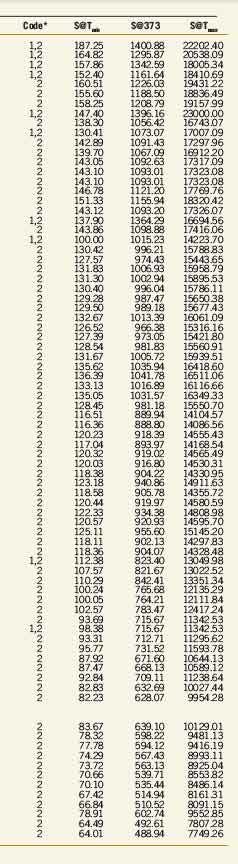
The accompanying table (see p. 98) gives the results for solubility of water in cycloalkanes as a function of temperature. The tabulation is arranged by carbon number (C5, C6, C7, ....) to provide ease of use in quickly locating data that use the chemical formula.
The tabulated values are based on both experimental data and estimates. In the absence of data, the estimates for isomers and large compounds (compounds with more than 10 carbons, i.e., compounds larger than C10) should be considered rough values, useful for initial analysis. If initial analysis is favorable, follow-up experimental determination is recommended.
Equation 2 was used to ascertain values for the estimates.
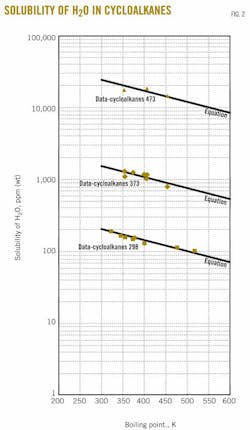
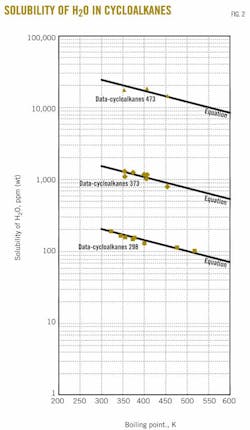
Fig. 2 shows solubility values obtained from the estimation equation and experimental data for three temperatures (298.15, 373.15, and 473.15 K). The data in the figure are based on the compilations of Englin et al.,2 Solubility Data Series,4-10 Sorensen and Artl 11 and Yaws19 at ambient temperature and Marche et al.3 and Tsonopoulos et al.12-14 at higher temperatures.
Boiling point temperatures are from the compilations of Yaws.16-18 General agreement of equation and data appear for the curves at the three different temperatures.
Example
In hydrocarbon processing, cyclohexane (C6H12) comes into contact with water at 393.85 K (120.7° C). The organic and aqueous phases are subsequently separated.
Estimate the concentration of water in cyclohexane at this temperature. Substitution of the coefficients and temperature into the correlation equation yields the calculation shown in the box on this page.