How temp. affects H2O solubility in cycloalkanes
Carl L. Yaws
Urvashi Yadav
Lamar University
Beaumont, Tex.
The solubility of water in cycloalkanes (cyclopentanes and cyclohexanes) found in crude oil as a function of temperature has been calculated.
In addition, we have developed a new correlation for solubility of water in crude oil that provides reliable solubility values down to very low concentrations (ppm). The correlation is based on boiling point temperature of the cycloalkane. Correlation values and experimental data are in agreement.
The results are usable in engineering applications involving processing, safety, hazard, environmental, and environmental considerations.
Previous articles (OGJ, Mar. 2, 2009, p. 52; Feb. 1, 2010, p. 54) provided data for water solubility in hydrocarbons at ambient temperature. Another (OGJ, Dec. 6, 2010, p. 130) provided data for the effect of temperature on the solubility of water in alkanes.
The present article provides data for the effect of temperature on the solubility of water in cyclyoalkanes. This article and the immediately previous one cover prominent alkanes and cycloalkanes contained in crude oil.
In refinery operations, many different temperatures are encountered in the processing of hydrocarbons in crude oil. This and the previous article provide data for the effects of temperature that are especially applicable for many different temperatures encountered in the processing of such hydrocarbons as alkanes and cycloalkanes in a refinery.
Water solubility
The importance of the solubility of water in hydrocarbons such as cyclyoalkanes in crude oil will increase in view of processing, safety, hazard, environmental, and environmental considerations focusing on product quality and equipment sustainability. The following brief discussion illustrates the importance.
Any processing that lowers temperature to near the freezing point of water may result in the formation of solids (freezing of water or hydrate formation). Such formation will affect both fluid flows in piping and operational characteristics of equipment. For catalytic reactions, any water in the hydrocarbon may poison the catalyst that promotes the hydrocarbon reaction.
For reactions in general, any water in the reaction species may result in the formation of undesirable by-products issuing from the hydrocarbon reaction. The presence of water in the product may degrade quality and, if sufficient water is in the product, it may prove to be unusable by the customer.
This brief discussion indicates that solubility of water in hydrocarbons contained in crude oil is important in engineering applications involving processing, safety, hazard, environmental, and environmental considerations.
Correlation
The correlation for water solubility as a function of temperature is based on Equation 1 in the accompanying equations box. The correlation applies to a range of about 298 to 478 K.
The coefficients (A, B, and C) for the correlation were determined from regression of the available data. In preparing the correlation, we conducted a literature search to identify data source publications.1-19
We screened the publications and copied appropriate data. These data were then keyed into the computer to provide a data base for which experimental data are available. The database also served as a basis to check the accuracy of the correlation.
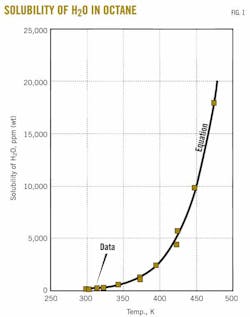
Fig. 1 shows the solubility of water as a function of temperature for a representative cycloalkane (cyclohexane). For the graph, we selected the data of Englin et al.,2 Marche et al.,3 and Tsonopoulos and Wilson.12 Fig. 1 discloses favorable agreement of data and equation.
Tabulation, estimation of equation
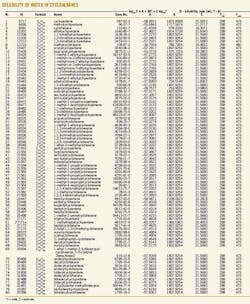
The accompanying table (see p. 98) gives the results for solubility of water in cycloalkanes as a function of temperature. The tabulation is arranged by carbon number (C5, C6, C7, ....) to provide ease of use in quickly locating data that use the chemical formula.
The tabulated values are based on both experimental data and estimates. In the absence of data, the estimates for isomers and large compounds (compounds with more than 10 carbons, i.e., compounds larger than C10) should be considered rough values, useful for initial analysis. If initial analysis is favorable, follow-up experimental determination is recommended.
Equation 2 was used to ascertain values for the estimates.
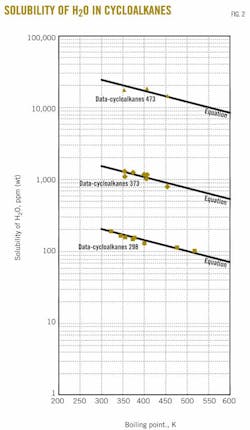
Fig. 2 shows solubility values obtained from the estimation equation and experimental data for three temperatures (298.15, 373.15, and 473.15 K). The data in the figure are based on the compilations of Englin et al.,2 Solubility Data Series,4-10 Sorensen and Artl 11 and Yaws19 at ambient temperature and Marche et al.3 and Tsonopoulos et al.12-14 at higher temperatures.
Boiling point temperatures are from the compilations of Yaws.16-18 General agreement of equation and data appear for the curves at the three different temperatures.
Example
In hydrocarbon processing, cyclohexane (C6H12) comes into contact with water at 393.85 K (120.7° C). The organic and aqueous phases are subsequently separated.
Estimate the concentration of water in cyclohexane at this temperature. Substitution of the coefficients and temperature into the correlation equation yields the calculation shown in the box on this page.
References
1. Black, C., et al., "The Solubility of Water in Hydrocarbons," Journal of Chem. Phy., Vol. 16 (1948), pp. 537-43.
2. Englin, B.A., et al., "Solubility of Water in Individual Hydrocarbons," Khim. Tekhnol. Topl. Massel, Vol. 9 (1965), p. 42.
3. Marche, C., et al., "Apparatus for the Determination of Water Solubility in Hydrocarbon: Toluene and Alkylcyclohexanes (C6 to C8) from 30 to 180 C," Journal of Chemical Engineering Data, Vol. 51 (2006), pp. 355-59.
4. Ng, H.J., and Chen, C.J., "Mutual Solubility in Water-Hydrocarbon Systems," Tulsa: Gas Processors Association Research Report 150, 1995.
5. Solubility Data Series, International Union of Pure and Applied Chemistry, Vol. 37, Hydrocarbons with Water and Seawater, Part 1—Hydrocarbons C5 to C7, Oxford, UK: Pergamon Press, 1989.
6. Solubility Data Series, International Union of Pure and Applied Chemistry, Vol. 38, Hydrocarbons with Water and Seawater, Part 1—Hydrocarbons C8 to C36, Oxford, UK: Pergamon Press, 1989.
7. Solubility Data Series, International Union of Pure and Applied Chemistry, Vol. 81, D.G. Shaw and A. Maczynski, editors, Hydrocarbons with Water and Seawater—Revised and Updated,. Part 9, C10 Hydrocarbons with Water, Journal of Physical Chemistry Ref. Data, Vol. 35 (2006), pp. 93-151.
8. Solubility Data Series, International Union of Pure and Applied Chemistry-NIST, D.G. Shaw and A. Maczynski, editors, Vol. 81. Hydrocarbons with Water and Seawater—Revised and Updated. Part 10. C11 and C12 Hydrocarbons with Water, Journal of Physical Chemistry. Ref. Data, Vol. 35 (2006), pp. 153-204.
9. Solubility Data Series, International Union of Pure and Applied Chemistry-NIST, D.G. Shaw and A. Maczynski, editors, Vol. 81. Hydrocarbons with Water and Seawater—Revised and Updated. Part 11. C13-C36 Hydrocarbons with Water, Journal of Physical Chemistry. Ref. Data, Vol. 35 (2006), pp. 687-784.
10. Solubility Data Series, International Union of Pure and Applied Chemistry-NIST, D.G. Shaw and A. Maczynski, editors, Vol. 81. Hydrocarbons with Water and Seawater—Revised and Updated. Part 12. C5-C26 Hydrocarbons with Seawater, Journal of Physical Chemistry. Ref. Data, Vol. 35 (2006), pp. 785-838.
11. Sorensen, J.M., and Artl, W., Liquid Liquid Equilibrium Data Collection, Vol. V, Part 1, Frankfurt: Dechema Chemistry Data Series, 1979.
12. Tsonopoulos, C., and Wilson, G.M., "Mutual Solubilities of Hydrocarbons and Water—Part I: Benzene, Cyclohexane, and n-Hexane," AIChE Journal, Vol. 29 (1983), pp. 990-999.
13. Tsonopoulos, C., et al., "Mutual Solubilities of Hydrocarbons and Water—Part II: Ethylbenzene, Etyhlcyclohexane, and n-Octane," AIChE Journal, Vol. 31 (1985), pp. 376-84.
14. Tsonopoulos, C., et al., "Mutual Solubilities of Hydrocarbons and Water—Part III: 1-Hexene, 1-Octene, and C10 to C12 Hydrocarbons," AIChE Journal, Vol. 43 (1997), pp. 535-46.
15. Tsonopoulos, C., "Thermodynamic Analysis of the Mutual Solubilities of Hydrocarbons and Water," Fluid Phase Equilibrium, Vol. 186 (2001), pp. 185-206.
16. Yaws, C.L., Chemical Properties Handbook, New York: McGraw-Hill Inc., 1999.
17. Yaws, C.L., Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, Houston: Gulf Publishing Co., 2005.
18. Yaws, C.L., Yaws Handbook of Vapor Pressure-Antoine Coefficients, Houston: Gulf Publishing Co., 2007.
19. Yaws, C.L., Yaws Handbook of Properties for Environmental and Green Engineering, Houston: Gulf Publishing Co., 2008.
20. Yaws, C.L., et al., "Table, correlation give water solubility, Henry’s Law constant for alkanes in crude," Oil & Gas Journal, Mar. 2, 2009, p. 52.
21. Yaws, C. L., et al., "Calculating Water solubility, Henry’s Law Constant for Cycloalkanes in Crude," Oil & Gas Journal, Feb. 1, 2010, p. 54.
22. Yaws, C. L. et al., "How temperature affects H2O solubility in alkanes," Oil & Gas Journal, Dec. 6, 2010, p. 130.
The authors
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com