Nuclear energy proposed for production of shale oil
Nuclear energy is being proposed as an alternative to gas-powered electricity as a heat source for in situ shale oil recovery in the US.
Nuclear engineer Charles W. Forsberg of the US Department of Energy’s Oak Ridge National Laboratory, Oak Ridge, Tenn., described the proposal at the American Nuclear Society’s 2006 International Congress on Advances in Nuclear Power Plants in Reno, Nev., June 7.
He said nuclear heat could be used to extract the vast US oil shale deposits more economically and in a much more environmentally benign manner than traditional oil shale recovery methods. Forsberg is senior scientist and senior reactor technical advisor for the lab’s Nuclear Science and Technology division.
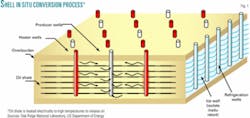
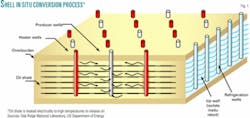
The technology is a modification to Shell Oil Co. new cost-effective “In Situ Conversion Process,” a retorting method that it has been developing since the 1980s. The method involves “drilling wells into oil shale, using electric heaters to raise the bulk temperature of the oil shale deposit to about 370° C. to initiate chemical reactions that produce light crude oil, and then pumping the oil to the surface,” Forsberg told ANS.
This process has been tested on a small scale and is now being scaled up to a precommercial size at a potential production cost of about $30/bbl-less than half that required for traditional oil shale recovery processes, Forsberg said. However, the company is still addressing some major technical challenges, he added.
The alternative to electric heat is to recover oil from shale using direct heat provided by a nuclear reactor. This would release lower volumes of greenhouse gases, particularly carbon dioxide, and cost less than traditional in situ methods.
The US has 500 billion-1.1 trillion bbl of potentially recoverable shale oil in the Green River, Washakie, Uinta, and Piceance Creek basins in Colorado, Utah, and Wyoming, Forsberg said.
Because most US oil shale deposits are more than 500 ft thick-with some more than 2,000 ft thick-some areas of the basins can yield more than 2.5 million bbl/acre of oil, he said, and usable deposits contain 25-50 gal/ton of oil shale.
Traditional recovery
Oil shale has a low thermal conductivity, requiring heater wells operating at high temperature to achieve the required medium temperatures in the bulk oil shale within 2-3 years.
Creating high-temperature electric heating for these wells constitutes the primary production cost in shale oil recovery. The usual energy source is fossil fuel, the combustion of which releases large amounts of CO2.
In the traditional recovery process, companies burn oil shale by injecting oxygen and heating the shale rapidly at more than 480° C. (900° F.). The process can be conducted above ground in retort vessels or in situ. In underground operations, a volume below the retort zone is mined, staged explosives break up or “rubblize” the shale to be retorted, and air is pumped in to burn some of the carbon to produce heat.
The initial cost of operating first-generation commercial plants of this type is expected to be $70-95/bbl, which Forsberg said could drop to $30-40/bbl over the long term with industrial experience.
In addition to being costly, this process produces low quality, unstable oil that requires major refining.
Shell’s process
The cost and potential environmental impact of the Shell in situ conversion process are much less than those for other shale oil processes, coal liquefaction, and other alternative methods of producing oil.
The system utilizes 15-25 electrical heaters/acre placed in vertical boreholes that heat the oil shale for 2-3 years to 370º C. (650-700° F.; Fig. 1). This slow heating and relatively low temperature cause the rock to release its oil and a gas similar to natural gas.
The produced oil is a high-quality, stable, light crude that requires little refining, and the gas requires minimal processing because it does not contain large amounts of CO2 or nitrogen from burning organics in the shale. About two thirds of the energy content is oil, and one third is gas.
The primary environmental impact in this method is electricity production. Downhole heating requires 250-300 kw-hr of electricity/bbl of oil. Gas from the shale, accounting for about one third of the energy content of the hydrocarbons produced, could be used to generate the electricity. But at a conversion efficiency of 60% all the produced gas would have to be dedicated to this use. Forsberg said lower-cost coal is a more likely energy source.
“There are complex tradeoffs between the number of heater wells, peak heater-well temperature, product yield, and product characteristics,” said Forsberg. Variables affecting decisions include shale oil’s low thermal conductivity, the need to heat the bulk rock for several years, and the use of well heating vs. bulk heating.
“These imply peak heater-well temperatures of 600-1,000º C. with incentives for the higher temperatures to reduce the number of heaters or reduce the heating time,” Forsberg said.
Shell uses freeze walls, an existing industrial technology, to isolate the underground retort from the geological formation. A sealed ice wall is formed by drilling wells around the perimeter of the extraction zone and using cooling coils to freeze the groundwater.
The freeze wall allows dewatering of the oil shale; avoids in-leakage of groundwater; keeps light hydrocarbon products from escaping during ground heating, product extraction, and post-extraction ground cooling; and allows post-treatment of the impacted rock zone to minimize the potential for long-term groundwater contamination.
Nuclear advantages
According to Forsberg, a high-temperature nuclear reactor could heat shale directly, eliminating the need for electricity from fossil fuel and avoiding the energy loss in converting heat to electricity and then back to heat. This would reduce the energy requirements for retorting the oil shale by a factor of 2 and avoid CO2 and other emissions as well from the production of electricity.
Consequently, the CO2 releases per unit of oil will be much less than those for other methods used to produce a synthetic crude oil. In addition, more oil can be produced because in situ volumes would not be required to heat the shale.
The nuclear process also requires less water than traditional extraction methods.
Nuclear heat for oil shale is potentially viable, Forsberg said, even though transfer of heat with heat transfer loops is limited to short distances, because the thick oil-shale deposits are “the most concentrated fossil resource on the planet.” This concentration makes it practical to build a reactor and operate it for decades while limiting the heat transport distance to a few kilometers.
Nuclear process heat
The proposed alternative process would use such nuclear reactors to provide the heat required to retort the oil shale.
Nuclear reactors, which are capital-intensive with low operating cost and good economics, require long-term base-load operations.
Production of 100,000 b/d of oil in the thickest oil shale deposits of the Piceance basin requires oil recovery from 30 acres/year and a reactor with a thermal output of 600 Mw. Heat can be transported for several kilometers, and the distances from reactor to wellhead are short enough to be practical. At full use of the oil shale, including the area under the reactor, in 40 years the longest heat-transport pipeline from reactor to wellhead would be less than 1,200 m.
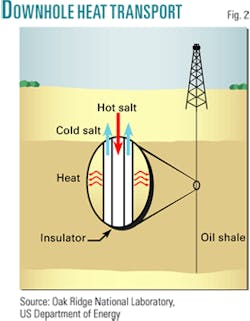
The downhole heat transport nevertheless presents a key challenge in effectively transporting heat at high temperatures over a considerable distance and down a well that may be 1,000 m deep. For nuclear heating, kilometer-long heater wells must be drilled with insulated inner pipe for the downward circulating heat-transfer fluid. In effect, a large bayonet heater is created (Fig. 2).
For the heat transfer fluid, chemically stable liquid-metal or liquid-salt heat-transport fluids are required that have a high volumetric heat capacity, high boiling point, low pumping costs, and low pressures. These will minimize the diameter of the wells and ensure efficient heat transfer between the coolant and well casing.
“The heat transfer coefficients of gases compared to liquids are much lower and thus limit heat transfer from the coolant to the well casing,” said Forsberg. “The low volumetric heat capacity of gases relative to liquids implies much larger pipes to transfer the same amount of heat.” The pumping costs for gases also would be much higher, he said.
Some of the candidate salts, such as fluorides and chlorides, are shown in Table 1. There are three major classes: halide salts, nitrate salts, and liquid metals. Values for water are also shown for comparison.
The halide salts are excellent high-temperature coolants with high volumetric heat capacities (ρCp), a critical requirement to enable transfer of large quantities of heat down a well.
Several fluoride salts are being considered. Although fluoride salts have been developed as heat transfer fluids, they have not been deployed commercially, Forsberg said. Fluoride salts are thermodynamically stable at high temperatures and have very high boiling points. Liquid fluoride salts do not react with helium or nitrogen but will react slowly with water. These salts have been injected into water with no violent steam explosion or chemical reaction.
The other potential halide salt systems are the chloride salts. Selected chloride salts have slightly lower melting points-355º C. for lithium chloride and potassium chloride (LiCl-KCl)-than some of the fluoride candidates, but little work has been done testing the use of these salts for industrial systems.
Inexpensive nitrate salts have been used as heat transfer fluids for about 80 years, but they may not meet the minimum temperature requirements, as they decompose near 600° C. They also could have exothermic reactions with organics.
Relatively inexpensive alkali metals such as sodium also have been used for decades as heat transfer fluids, but they have lower volumetric heat capacities (higher volume flow rates per well) and have highly exothermic reactions with water and air, said Forsberg.
Technical options
In addition to the selection of effective coolant-material combinations, the development of start-up and shut-down procedures for the heater wells presents technical challenges. Each option has advantages, disadvantages, and challenges, said Forsberg
Start-up options to avoid freezing the coolant in the cold well during initial operations include:
- Preheating the well with hot gases or electric heat.
- Filling the well with the cold solid coolant in particulate form and then slowly lowering the center feed pipe with hot circulating coolant that heats and melts the solid coolant as the feed pipe is lowered to the bottom of the well.
- Two coolant combinations such as initial heating with a lower temperature coolant such as condensing steam or nitrate salts and then switching to a higher temperature coolant after initial heating of the oil shale.
Reactor options
High-temperature reactors used in oil shale recovery would use graphite-matrix coated-particle fuel with one of two potential coolants: helium or liquid fluoride salts. Modular gas-cooled reactors are the near-term technical option because several such reactors have been built, said Forsberg
The longer-term option is the advanced high temperature reactor (AHTR), also known as the liquid-salt-cooled, very-high-temperature reactor. The AHTR has potential economic advantages but is in an early stage of development, he said.
The AHTR has one unique advantage in the context of oil shale recovery, however. “It is a low-pressure, liquid-salt-cooled reactor with a small pressure drop across the reactor core. In liquid-cooled reactors, the temperature rise across the reactor core can be selected to match the required temperature rise for the intermediate heat transfer fluid through the intermediate heat exchanger,” explained Forsberg.
In gas-cooled reactors, the high-pressure drop across the reactor core and resultant cost of pumping typically result in a 350° C. temperature rise across the reactor core, he said. In many cases, it will be difficult to match the temperature rise in the reactor core to the desired temperature rise of the heat transfer loop in the intermediate heat exchanger.