P. 2 ~ Continued - Mixing MDEA, TEA shows benefit for gas-sweetening operations
View Article as Single page
Process chemistry, thermodynamics
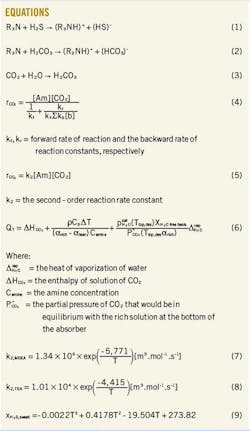
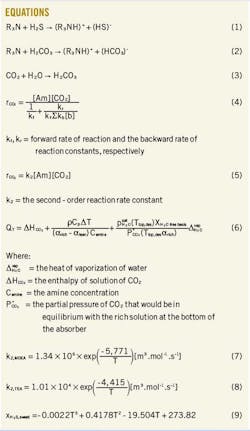
Since both MDEA and TEA are tertiary amines, this article is more concerned with the reaction mechanism associating these types of solvents. Equations 1-3 (in the accompanying equations box) are the reactions taking place in the absorption section.2 These reactions are driven by high pressure and low temperature. Reverse reactions take place in the regenerator, and they are favored by low pressure and high temperature.
Tertiary amines do not form carbamates as a result of their reaction with CO2. They only catalyze the slow hydrolysis of CO2 to form bicarbonates.3 This is a principal difference between them and any primary or secondary amine solvents. For that reason, MDEA and TEA are considered to be "selective" due to their rapid rate of reaction with H2S to form heat-stable salts.
Traditionally, the main objective of mixing primary or secondary amines with tertiary amines is to combine between higher CO2 reaction rates of primary and secondary amines with the higher CO2 loading capacity of tertiary amine.4 As a result, this type of blend is mainly used for bulk removal of acid gas. In addition, solvents with higher reactivity as indicated by higher forward reaction rate constants (primary and secondary amines) also tend to have higher heats of reaction.
CO2 reacts reversibly with primary and secondary amines through a zwitterion mechanism to form an amine carbamate. Initially, CO2 reacts with the amine to form a carbamate, followed by extraction of the proton through bases present in the solution. The rate of reaction for primary or secondary amines can be written as Equation 4.5
Tertiary amines do not form a carbamate due to the absence of hydrogen attached to the nitrogen atom.4 Instead, they react in a single step mechanism resulting in the formation of a protonated amine and a bicarbonate ion. The rate of reaction for tertiary amines can be written as shown in Equation 5.5
Studies by Bell and Trotman-Dickenson and Trotman-Dickenson on the decomposition of nitramide showed that amines are effective as base catalysts.6 7 Also, Sharma demonstrated that a correlation for CO2 kinetic constants and base strength applies.8 Table 1 shows second-order rate of reaction constants for various alkanolamine solvents with CO2 at 25o C.9 10 It is obvious that base strength decreases as the reaction rate decreases from primary through secondary to tertiary amines.
Absorption carried out with a chemical solvent involves mass transfer with a series of exothermic reactions. The role of the chemical reaction is to speed up the mass-transfer rate as well as to provide greater solvent loading capacity at lower CO2 partial pressure conditions. For the stripper, the reversible reaction is endothermic and favored under high temperature and low partial pressure conditions.
Therefore, solvents with higher heat of reaction with CO2, due to the carbamate reaction, will require more energy for regeneration, and they tend to have higher lean amine loadings (αlean) than the less reactive ones.11 According to Rochelle, the total energy requirement to regenerate the absorbent (QT) in a stripper depends substantially on the heat of reaction (∆ HCO2) and can be mathematically expressed with Equation 6.12 13
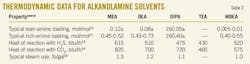
Table 2 shows typical thermodynamic data for various alkanolamine solvents. Heats of reactions between amine, H2S, and CO2 are given at 100o F.14 15 16 As shown, tertiary amines hold the lowest lean loading as well as the lowest steam use for regeneration process. Also, it is illustrated how heat of reaction varies, depending on the chemical structure of the alkanolamine. Tertiary amines produce the lowest heat of reaction with acid gases, which makes it attractive energy-wise.
Process simulation results obtained for MDEA-TEA mixture in comparison with other known alkanolamine mixtures can be explained based on reaction mechanism, kinetic, and thermodynamic data presented earlier. The total solvent concentration of MDEA plus the additive is always fixed to 45 wt %. Table 3 shows the loading capacities absorbents at 0.66 bars and 75o C.17 As shown, MDEA has higher loading capacity than TEA for both H2S and CO2.
Displaying 2/6
View Article as Single page