Mixing MDEA, TEA shows benefit for gas-sweetening operations
Wael A. Fouad
Abdallah S. Berrouk
Cornelis J. Peters
Petroleum Institute
Abu Dhabi
Studies have demonstrated how mixing primary-tertiary or secondary-tertiary amines have improved the efficiency of amine-sweetening processes in terms of gas purification and process energy requirements. This article discusses the use of amine solvents that consist of two tertiary amines, methyl-diethanolamine (MDEA) and triethanolamine (TEA).
A kinetic approach was used to explain results obtained from a process simulation of the Habshan gas sweetening unit in Abu Dhabi. Results show that up to a 3% reduction in unit running cost can be obtained with a mixture consisting of 40 wt % MDEA and 5 wt % TEA), while meeting sweet-gas specifications in terms of H2S and CO2 concentrations. Lean amine loading was fixed at a value of 0.005.
Results for the MDEA + TEA mixture were compared with results of the standardized (45 wt % MDEA) solvent used in Habshan and other possible primary-tertiary and secondary-tertiary amine mixtures. This reduction in cost was achieved through a decrease in plant raw materials cost and in both regenerator reboiler and trim-cooler energy requirements.
Sweetening processes
New technologies are being implemented recently to treat highly sour natural gas with different gas sweetening processes. As the gas acidity increases, the energy required by the process to achieve sweet gas specifications in terms of H2S and CO2 concentrations will increase. This is particularly true for gas sweetening processes that use such alkanolamine solvents as the primary amine methyl-ethanolamine (MEA), the secondary amines diethanolamine (DEA) and di-isopropanolamine (DIPA), and the tertiary amines triethanolamine (TEA) and methyl-diethanolamine (MDEA).
Many recent researches have focused on the mixing of different amine solvents and how their use affects both acid gas components' absorption and the energy price of the process. So far as the authors know, all these investigations have been on mixing primary and tertiary amines (MEA-MDEA, for instance) or secondary and tertiary amines (DEA-MDEA, for example).
The main reason behind mixing primary or secondary amines with tertiary is to benefit the sweetening process from the high reactivity of the former and the low energy requirement of the latter. Other reasons can also be mitigation of the effects of the relatively higher vapor pressure and corrosion rates of the primary and secondary amines as well as the relatively higher selectivity of tertiary amines towards H2S.
Usually, process simulations have been used to determine the proportionality of these different amines in the mixed solution that can meet the acid-gas-removal specification at the lowest energy requirement possible. The latter is estimated to be about 70% of the operating costs of an amine gas-sweetening unit, excluding labor.1 Thus, every option available for the process industry to lower the energy requirement should be investigated, in particular for sour gas feeds.
Abu Dhabi Gas Industries Ltd. (GASCO) has been running the Habshan sweetening gas units that treat gas streams with up to 10% of H2S. Newly developed gas reserves in Abu Dhabi are even more sour, with H2S concentrations reaching 30%. The amine used in these units has been MDEA at 45 wt %. This tertiary amine is known for its low vapor pressure and thus can be used at higher concentrations (up to 55 wt %) without appreciable vaporization losses for acid-gas removal from high-concentration natural gas.
MDEA is known for its selectivity towards H2S and its low energy requirement for regeneration compared with primary and secondary amines. However, MDEA slips some of the CO2, which can be a drawback only if CO2 specifications are strict. Triethanolamine (TEA) is another tertiary amine that has a lower energy requirement than MDEA but a lower loading capacity as well.
This article discusses the effect of a mixture of two tertiary amines, MDEA and TEA, on the performance of the Habshan gas-sweetening unit belonging to the onshore gas development (OGD) project Phase II. This unit is treating a sour gas feed with 4.26% mol of H2S and 5.34% mol of CO2. Performance is measured in terms of acid-gas removal and running cost. Enthalpies, kinetics, and acid gas loadings are used to examine the effect of such blend on the unit performance.
Process chemistry, thermodynamics
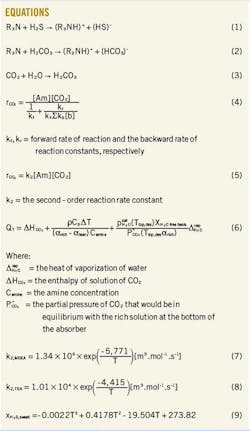
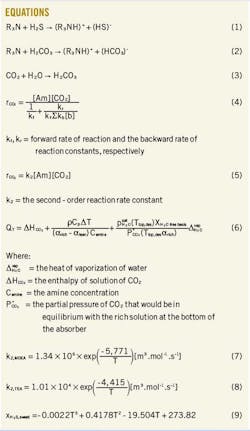
Since both MDEA and TEA are tertiary amines, this article is more concerned with the reaction mechanism associating these types of solvents. Equations 1-3 (in the accompanying equations box) are the reactions taking place in the absorption section.2 These reactions are driven by high pressure and low temperature. Reverse reactions take place in the regenerator, and they are favored by low pressure and high temperature.
Tertiary amines do not form carbamates as a result of their reaction with CO2. They only catalyze the slow hydrolysis of CO2 to form bicarbonates.3 This is a principal difference between them and any primary or secondary amine solvents. For that reason, MDEA and TEA are considered to be "selective" due to their rapid rate of reaction with H2S to form heat-stable salts.
Traditionally, the main objective of mixing primary or secondary amines with tertiary amines is to combine between higher CO2 reaction rates of primary and secondary amines with the higher CO2 loading capacity of tertiary amine.4 As a result, this type of blend is mainly used for bulk removal of acid gas. In addition, solvents with higher reactivity as indicated by higher forward reaction rate constants (primary and secondary amines) also tend to have higher heats of reaction.
CO2 reacts reversibly with primary and secondary amines through a zwitterion mechanism to form an amine carbamate. Initially, CO2 reacts with the amine to form a carbamate, followed by extraction of the proton through bases present in the solution. The rate of reaction for primary or secondary amines can be written as Equation 4.5
Tertiary amines do not form a carbamate due to the absence of hydrogen attached to the nitrogen atom.4 Instead, they react in a single step mechanism resulting in the formation of a protonated amine and a bicarbonate ion. The rate of reaction for tertiary amines can be written as shown in Equation 5.5
Studies by Bell and Trotman-Dickenson and Trotman-Dickenson on the decomposition of nitramide showed that amines are effective as base catalysts.6 7 Also, Sharma demonstrated that a correlation for CO2 kinetic constants and base strength applies.8 Table 1 shows second-order rate of reaction constants for various alkanolamine solvents with CO2 at 25o C.9 10 It is obvious that base strength decreases as the reaction rate decreases from primary through secondary to tertiary amines.
Absorption carried out with a chemical solvent involves mass transfer with a series of exothermic reactions. The role of the chemical reaction is to speed up the mass-transfer rate as well as to provide greater solvent loading capacity at lower CO2 partial pressure conditions. For the stripper, the reversible reaction is endothermic and favored under high temperature and low partial pressure conditions.
Therefore, solvents with higher heat of reaction with CO2, due to the carbamate reaction, will require more energy for regeneration, and they tend to have higher lean amine loadings (αlean) than the less reactive ones.11 According to Rochelle, the total energy requirement to regenerate the absorbent (QT) in a stripper depends substantially on the heat of reaction (∆ HCO2) and can be mathematically expressed with Equation 6.12 13
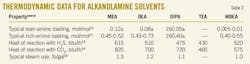
Table 2 shows typical thermodynamic data for various alkanolamine solvents. Heats of reactions between amine, H2S, and CO2 are given at 100o F.14 15 16 As shown, tertiary amines hold the lowest lean loading as well as the lowest steam use for regeneration process. Also, it is illustrated how heat of reaction varies, depending on the chemical structure of the alkanolamine. Tertiary amines produce the lowest heat of reaction with acid gases, which makes it attractive energy-wise.
Process simulation results obtained for MDEA-TEA mixture in comparison with other known alkanolamine mixtures can be explained based on reaction mechanism, kinetic, and thermodynamic data presented earlier. The total solvent concentration of MDEA plus the additive is always fixed to 45 wt %. Table 3 shows the loading capacities absorbents at 0.66 bars and 75o C.17 As shown, MDEA has higher loading capacity than TEA for both H2S and CO2.
Process simulation
Different alkanolamine mixtures were simulated with the TSWEET kinetic model incorporated in the process simulator ProMax 3.0 software.18 Table 4 presents design data used for simulations for the Habshan gas unit OGD II. They were provided by GASCO.
The additive concentration (MEA, DEA, DIPA, or TEA) was varied from 0 to 20 wt % with the solvent total amine concentration fixed at 45 wt % for all the studied cases. (MDEA concentrations vary between 45 and 25 wt %.) For the first simulations, convergence of the regenerator was obtained through specification of a condenser temperature of 57o C. and a lean amine loading of 0.005 total moles of acid gas/moles of amine. Then, a final case study was carried out to investigate the effect of varying the lean amine loading on the reduction of the reboiler duty.
Results obtained for the mixture MDEA-TEA were compared in terms of performance with the other currently used alkanolamine mixtures such as MDEA-MEA, MDEA-DEA, and MDEA-DIP, and also against results obtained for the use of MDEA only. (GASCO Habshan unit OGD II uses only MDEA solvent at 45 wt %.)
Comparison between different mixtures was made based on regenerator reboiler duty, H2S and CO2 concentration in sweet gas, and rich amines' loading capacities. Also, the effects of changing the trim-cooler temperature on the absorption of acid gas by MDEA-TEA mixture were studied in order to achieve the initial H2S specification produced by the single MDEA solvent 45 wt %. Finally, the economic impact of the use of MDEA-TEA mixture (40 wt % MDEA + 5 wt % TEA) on the unit's operating costs was discussed.
Results, discussion
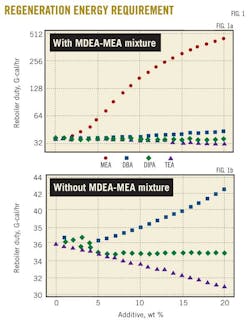
Fig. 1 shows the regenerator reboiler duty necessary to strip different rich-amine solutions from acid gases as function of the additive percentage in the 45 wt %. MDEA-X. X stands for MEA, DEA, DIPA, and TEA solutions with a percentage varying from 0 to 20 wt %.
Fig. 1a shows the dramatic increase of the stripper's energy requirement with the increase in the MEA contribution to the 45 wt % MDEA-MEA amine mixture. Results of Fig. 1a are also depicted in Fig. 1b but without those for MDEA-MEA mixture. The reason is to show the difference in energy requirements for MDEA-DEA, MDEA-DIPA, and MDEA-TEA mixtures.
As far as the addition of DIPA is concerned, a small increase and decrease in the regenerator duty occurs within the 5% addition of DIPA. Beyond this percentage of added DIPA, the stripper energy requirement becomes insensitive to the DIPA quantity used to replace MDEA in the 45 wt % MDEA-DIPA mixture. For the MDEA-DEA mixture, an increase of 16% in the duty appears when 20% of the MDEA is replaced by DEA in the 45 wt % MDEA-DEA mixture.
Fig. 1b shows that addition of TEA has resulted in a constant decrease of the regenerator duty to 14% when 20% of the MDEA is replaced by TEA in the 45 wt % MDEA-TEA solution. This can be explained based on the data provided in Table 2, which shows that TEA had the lowest heat of reaction with H2S and CO2 compared with other solvents.
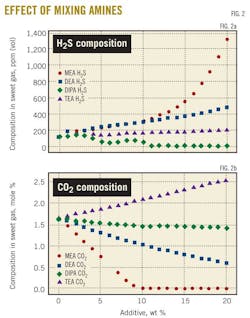
Fig. 2 depicts the effect of mixing of different amines with MDEA on the absorption of the acid gases.
Fig. 2a shows that TEA addition to MDEA causes an increase in H2S concentration in the sweet gas. DEA-MDEA and DIPA-MDEA mixtures, however, score higher H2S concentrations.
In terms of CO2 absorption, Fig. 2b shows that MDEA-TEA mixture is the worst among all the mixtures in terms of bulk removal of CO2. It is a matter of fact that all alkaline solutions are thermodynamically selective to CO2 and kinetically selective to H2S.
Table 1 shows that primary amines have the highest rate of reaction constants followed by secondary then by tertiary amines. When H2S dissolves in amine, it immediately converts to sulfide and bisulfide ions via an instantaneous and reversible protonation reaction with hydrogen ions.
Conversely, CO2 reacts more slowly than H2S and forms stable reaction products that do not depend on alkalinity, as it is the case for H2S ones. Kinetically, if both amine and acid gas were exposed to each other for a short residence time, H2S will react more rapidly than CO2 due to its instantaneous reaction that keeps the unreacted form of H2S low in the liquid phase, which helps to maintain high driving force. A different scenario, however, occurs if the CO2 reaction rate was high enough to prevent H2S concentration buildup. Higher absorption rate of CO2 will consume the amine and reduce its alkalinity.
This trend is maintained until the solvent alkalinity is too low to keep all H2S in a protonated form. Consequently, the solvent favors CO2 products that are driven through an almost irreversible reaction and allows the de-protonation of HS.– and desorption of H2S.19 As discussed earlier, primary and secondary amines have higher CO2 rates of reaction constants than TEA, hence better prevention of H2S concentration buildup.
These facts are well depicted by both Fig. 2a, where such mixtures as DEA-MDEA and DIPA-MDEA produced sweet gas with higher H2S contents than that produced by the tertiary-amine mixture MDEA-TEA. This analysis can also be used to explain the dramatic increase in the reboiler duty for MDEA-MEA mixture. MEA rate of reaction constant is about 1,200 times that of MDEA, which testifies to its high ability fully to absorb CO2 at a given short residence time. Literature review done by Ma'mun shows that CO2 partial pressure with 30 wt % MEA at a temperature range of 25-120o C. is measured to be in the range of 0.2-6616 kPa compared with 0.00161-6570 kPa for a solution of MDEA 50 wt % at the same temperature.20
Comparison between pure MDEA solvent and MDEA-TEA mixture can be made based on the acid-gas loading capacity. Table 1 shows that MDEA and TEA kinetics are close due to their similar chemistry. Table 4, however, demonstrates that MDEA has a higher acid-gas loading capacity than TEA. This explains better why MDEA-TEA mixture absorbs less H2S and CO2 than pure MDEA solvent.
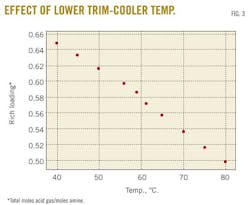
Different paths are available to take advantage of the reduced reboiler duty achieved by the mixture MDEA-TEA while keeping the sweet-gas specifications similar to those produced by single 45 wt % MDEA solvent. These paths include increasing the amine circulation rate or decreasing the trim-cooler temperature of 61o C. In this study, we chose optimization of the trim-cooler temperature because increasing the amine circulation rate is believed to be less cost effective due to the large difference between both solvents' loading capacities.
Goharrokhi shows data calculated with Chakma's correlation, in which it is obvious how the equilibrium constant in the MDEA protonation reaction gets higher with lower reaction temperature.21 In other words, decreasing the trim-cooler temperature shifts the equilibrium of the exothermic reaction forward, thus increasing the absorption rate. The absorption rate constant can be expressed mathematically with Equations 7 and 8 as functions of temperature for MDEA and TEA, respectively.10
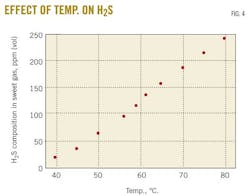
A case study predicted changes in the rich-amine loading by varying the trim-cooler temperature in the range 40 to 80o C. Fig. 3 demonstrates that decreasing the cooler temperature leads to an increase in rich-amine loading, thus a decrease in H2S and CO2 concentrations in sweet gas. Fig. 4 shows the change in H2S concentration in the sweet gas as function of temperature.
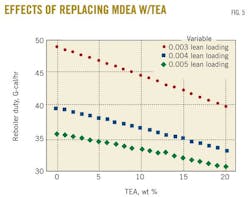
Finally, another case study verified the same conclusion can be drawn for different lean-amine loadings through quantifying their corresponding reboiler duties. Obviously, a lower lean loading requires a greater energy to regenerate the amine. This offers a stronger driving force through which absorption takes place and a sweeter gas product is obtained.
Fig. 5 shows that the same trend was obtained with different lean-amine loadings with almost equal slopes for lean-amine loading of 0.005 and 0.004. As the lean-amine loading decreases, a higher reduction in the reboiler duty is achieved. Indeed, for 20% of MDEA replaced by TEA, the reboiler duty reduction is calculated to be 16% and 18.5% when lean-amine loading passes from 0.004 to 0.003.
Economics
Solvent conversion or gas plant revamping is usually done in order to increase production or decrease plant operating cost. To confirm this practice, we conducted an economic feasibility analysis for OGD II gas sweetening unit to calculate the gross profit gained through the use of MDEA-TEA mixture (40 wt %. MDEA + 5 wt %. TEA) compared with the use of the pure MDEA solvent. GASCO provided the average raw material price for both MDEA and TEA. The power cost is assumed equal to the one used by GASCO's Habshan operating utilities, and amine solvent needed is equal to 30% of the fixed-roof storage tank volume used in the unit.
We derived a model (Equation 9) for the variation of the H2S concentration with trim-cooler temperature based on the results displayed in Fig. 5[4].
Adding 5 wt % TEA at 61o C. resulted in increasing H2S concentration in sweet gas from 121 to 136 ppm (vol). Simulations predict that a decrease of the temperature to 60o C. will reduce the H2S concentration to its initial value (the one obtained with pure MDEA solution 45 wt % and a trim-cooler temperature of 61o C.).
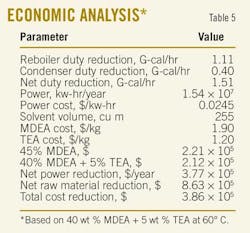
With this temperature, a reduction in the cooler duty is observed due to reduced reaction exothermicity, which lowered the absorber bottom stream temperature. Furthermore, this resulted into an inlet-cooler stream with a lower temperature and a reduction in the cooler duty of about 4.14%. Table 5 shows the approximate savings ($386,000/year) if an optimized process of adding 5 wt %. TEA and reducing the trim cooler temperature to about 60o C. is utilized.
Blending different types of amine solvents appear to benefit gas sweetening processes. This article has examined the effect of mixing two tertiary amines of MDEA and TEA on the amine sweetening unit's energy cost. Literature shows that TEA has a lower heat of reaction, lower CO2 rate of reaction, as well as lower acid-gas capacity compared to MDEA.
Comparison among different blends showed that MDEA-TEA mixture requires the lowest regeneration energy duty. Moreover, it still maintains the mixture selectivity due to the lower CO2 reaction rate and loading capacity which resulted in lower H2S content in sweet gas.
In comparison to single MDEA, TEA holds a smaller acid-gas loading capacity, which reduces the volume of acid gas it can absorb. Therefore, an investigation predicted the effect of altering the trim-cooler temperature in order to maintain the initial H2S concentration with single MDEA solvent. Results showed that about 3.0% reduction in running cost can be obtained for the OGD II unit.
An economic analysis calculated the money savings by utilizing (40 wt % MDEA + 5 wt % TEA) mixture to treat the sour feed gas. Results showed that up to $386,000/year/unit can be gained at a lean-amine loading of 0.005. Decreasing the latter has proved to lead to a further reduction in the required regeneration energy if the recommended tertiary amine mixture was used.
Acknowledgment
Financial support for this project was provided by the GASCO and Adgas operating companies of the Abu Dhabi National Oil Co.
References
1. Khakdaman, H.R., Zoghi, A.T., Abedinzadegan, M., and Ghadirian, H.A., "Revamping of gas refineries using amine blends," IUST Int. J. Eng. Sci., Vol. 19 (2008), pp. 27-32.
2. Cummings, A.L., Smith, G.D., and Nelsen, D.L., "Advances in amine reclaiming—why there's no excuse to operate a dirty amine system," Proceedings of Laurance Reid Gas Conditioning Conference, Norman, Okla., Feb. 27, 2007.
3. Darton, R.C., Distillation and Absorption Technology: Current Market and New Developments, Transactions of the Institution of Chemical Engineers, 1992; www.icheme.org.
4. Aliabadi, H.Z., and Mirzaei, S., "Using mixed amine solution for gas sweetening," W. Acad. of Sci. Eng. and Tech., Vol. 58 (2009), pp. 992-97.
5. Cullinane, J.T., "Thermodynamics and kinetics of aqueous piperazine with potassium carbonate for carbon dioxide absorption," Dept. Chem. Eng., University of Texas, Austin, topical report, Apr. 18, 2005.
6. Bell, R.P., and Trotman-Dickenson, A.F., "Acid-Base Catalysis in Non-Aqueous Solvents," Part XII. The Amine-Catalysed Decomposition of Nitramide in Anisole Solution, Journal Chem. Soc., Abs., 1949, pp. 1288-97.
7. Trotman-Dickenson, A.F. "The Basic Strength of Amines." Journal of Chem. Soc., Abs., 1949, pp. 1293-97.
8. Sharma, M.M., "Kinetics of Reactions of Carbonyl Sulphide and Carbon Dioxide with Amines and Catalysis by Bronsted Bases of the Hydrolysis of COS," Trans. Faraday Soc. Vol. 61 (1965), pp. 681-87.
9. Kuipers, J.A.M., Versteeg, G.F., Hogendoorn, J.A., van den Berg, H., Warmoeskerken, M.M.C.G., Geuzebroek, F.H., Feron, P.H.M., Turkenburg, W.C., and Svendsen, H.F., "Carbon dioxide absorption in piperazine activated n-methyldiethanolamine," PhD dissertation, University of Twente, the Netherlands, 2006, pp. 43-78.
10. Little, R.J., van Swaaij, W.P.M., and Versteeg, G.F., "Kinetics of Carbon Dioxide with Tertiary Amines in Aqueous Solution," AIChE Journal, Vol. 36 (1990), pp. 1633-40.
11. Veawab, A., Aroonwilas, A., Chakma, A., and Tontiwachwuthikul, P., "Solvent Formulation for CO2 Separation from Flue Gas Streams," Proceedings of the First National Conference on Carbon Sequestration, Washington, May 14-17, 2011, pp. 1-9.
12. Rochelle, G.T, Goff, G.S, Cullinane, J.T, and Freguia, S., "Research Results for CO2 Capture from Flue Gas by Aqueous Absorption/Stripping," Proceedings of Laurance Reid Gas Conditioning Conference, Norman, Okla., Feb. 25-27, 2002, pp. 131-51.
13. Ma'mun, S., Selection and Characterization of New Absorbents for Carbon dioxide Capture, Doctoral Thesis, Department of Chemical Engineering, Faculty of Natural Science and Technology, Norwegian University of Science and Technology, 2005.
14. Kohl, Arthur, and Nielsen, Richard, "Alkalnolamines for hydrogen sulfide and carbon dioxide removal,'' in Gas Purification, 5th edition, Houston: Gulf Publishing Co., 1997, pp. 41-56.
15. GPSA Engineering Data Book, 11th Edition, Tulsa: Gas Processors Suppliers Association, 1998.
16. Armstrong, T., and Gardner, A., "Refining details notebook, amine treating 2," Today's Refinery, April 1998, pp. 1-6.
17. Appl, M., Wagner, H.U., Henrici, K.J., Kuessner, K., and Volkamer, E.F., Removal of CO2 and/or H2S and/or COS from Gases Containing These Constituents, United States Patent, 1982.
18. Bryan Research & Engineering Inc., Powerful Process Simulation Technology (ProMax); http://www.bre.com.
19. Weiland, R.H., Oettler, B., Ender, C., and Dingman, J.C., "Selective amine treating using trays, structured packing, and random packing," Proceedings of the International Conference on Distillation and Absoprtion, Kongresshaus, Baden-Baden, Germany, September 2002.
20. Ma'mum, S., Nilsen, R., and Svendsen, H.F., "Solubility of carbon dioxide in 30 mass % Monoethanolamine and 50 mass % Methyldiethanolamine solutions," J. Chem. Eng. Data, Vol. 50, Jan. 28, 2005, pp. 630-34.
21. Goharrokhi, M., Taghikhani, V., Ghotbi, C., Safekordi, A., and Najibi, H., "Correlation and prediction of solubility of CO2 in amine aqueous solutions," Iran J. Chem. Eng., Vol. 29 (2010), pp. 111-24.
The authors
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com