Gas heated reforming improves Fischer-Tropsch process
Incorporating gas heated reforming (GHR) technology into autothermal reforming (ATR)-based GTL processes offers combined benefits of significantly higher carbon efficiency and lower capital cost.
The two reformers can be linked in different ways; the configuration in which feed gas passes through the GHR and ATR in series gives the best benefits.
Synetix, in partnership with Methanex Corp., the world's largest producer of methanol, has constructed a materials demonstration unit (MDU) in New Zealand to establish the benefits of combining GHR with ATR.
Engineering work on the project started in mid-2000, and the units were commissioned in Feb 2002. Preliminary results will be available later in 2002 with final results in 2003.
An important objective when making syngas feed for Fischer-Tropsch (FT) liquids production is to synthesize a gas composition in which the H2:CO ratio= 2:1. This is the approximate ratio in which these reactants are consumed.
Autothermal reforming
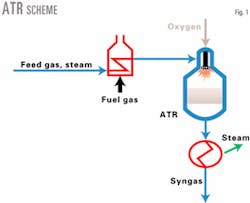
Currently, the most widely proposed syngas technology for GTL production is ATR. There are many variants of this basic process, some incorporating proprietary technology. All the processes, however, involve the partial oxidation of natural gas with steam and oxygen, or air, followed by equilibration over a catalyst bed (Fig. 1).
These catalytic processes have more ideal syngas stoichiometry for FT synthesis and lower capital cost compared to non-catalytic partial oxidation (POX).
With ATR, the feed gas and steam mixture is preheated, combusted with an oxidant, and passed over a catalyst to bring the steam-methane reforming reaction to equilibrium at a temperature of 1,790-1,920° F. The product gas is corrosive from 750° to 1,300° F. and must be cooled by creating steam in a waste-heat boiler.
For a constant ATR inlet temperature and steam ratio, a fixed amount of oxygen is combusted to heat the gases and steam reform them to equilibrium at 1,920° F. This satisfies the ATR molar reaction and adiabatic heat balance according to Eq. 1 (see Equation box).
This gives a syngas that is too lean in CO (H2:CO = 2.19) since CO2 is not reactive for FT. The CO2 can be recycled to the ATR, which will enable the correct syngas H2:CO ratio of 2.0 (Eq. 2).
The balance in Eq. 2 shows that there is a fundamental, inherent process inefficiency. For every 100 units of carbon in the feed, 9 units of carbon must be discarded to achieve a satisfactory FT feed composition.
Gas heated reforming
GHR was first developed by Synetix in the mid 1980s and has been in continuous operation in ammonia and methanol plants since 1988. In the 1990s, Synetix developed the advanced gas heated reformer (AGHR) with a simplified, lower-cost design and the ability to scale up to the sizes required for world-scale GTL plants.
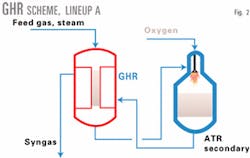
Combining the oxygen-fired reformer with a GHR can substantially improve the performance of a GTL plant using oxygen-based reforming.1 The GHR recycles high-grade heat from the reformed gas directly back into the reforming process. This reduces the oxygen requirement and eliminates the need to generate large quantities of high-pressure steam from the process train. Fig. 2 shows this configuration.
With the same basis as the preceding ATR example (natural gas composition, steam ratio, ATR and secondary exit temperature), 18% less oxygen combustion is needed to satisfy the heat balance and less hydrogen is consumed, leaving more available for FT liquid synthesis.
Eq. 3 shows that the scheme is now well balanced-for every 100 units of feed carbon, only 2 units of carbon must be purged, and reaction efficiency to CO improves nearly 8%. This will boost the overall carbon efficiency of a GTL plant.2
Economic benefits
We have completed detailed evaluations of the GHR technology. We compared the GHR-based process to POX and ATR in terms of process and utility requirements. Cost estimates are based on detailed equipment lists using established methods. The estimates include vendor quotes for major equipment items where appropriate.
The studies consistently show significant benefits with the GHR-based process. The main benefits are reduced:
- Total energy per barrel, up to 10%.
- Oxygen requirement, up to 25%.
- Overall CO2 emissions, up to 45%.
Table 1 summarizes the results of a study based on a light natural gas feedstock. The GHR-based process operating at a steam ratio (SR) of 2.0 achieves 5% lower total energy consumption than POX or ATR. If we lower the SR to less than 1.0, energy consumption improves by up to 10%.
The maximum efficiency improvement (and the SR at which it occurs) depends on many factors, such as feed composition, FT conversion and selectivity, LPG recovery, etc. The optimum is a balance between the feed and fuel requirements for natural gas; the optimum may be at an intermediate SR.
We configured all the processes for zero power export-any heat that could not be used in the process was rejected in flue gas or used for air or water cooling. Table 1 also shows the substantial reductions in oxygen requirement and CO2 emissions.
Capital costs
GHR has lower capital costs compared to competing processes. GHR simplifies the flowsheet, eliminating some systems and reducing the size and cost of others, especially regarding utilities. The main benefits are:
- Smaller air-separation unit.
- Elimination of the high-pressure process waste heat boiler.
- Simpler, smaller steam and power-generation systems.
- Lower cooling duties and smaller cooling systems.
At an SR of 2.0, capital cost is the same as the ATR-based process (at an SR of 0.6) and about 5% lower than for a POX-based system. At lower SRs, the capital cost savings are more than 10% for a complete GTL facility. Table 2 shows typical savings.
Different GHR configurations
There are various options for coupling a GHR with an oxygen-fired reformer. They require different process and mechanical design considerations and have different overall effects on GTL process performance.
In Lineup A (Fig. 2), the GHR and oxygen-based reformer (secondary reformer) are in series. GHR catalyst tube bottoms are sealed within a tubesheet to prevent methane leakage into the syngas product. This is the design of the Synetix AGHR.
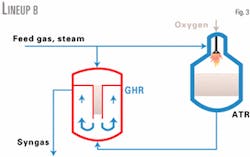
In Lineup B (Fig. 3), the natural gas and steam mixture is split and fed to the GHR and ATR, which operate in parallel. The GHR catalyst tubes are open ended. The two reformed gas streams mix in the GHR shell before they provide heat to reform the gas in the GHR.
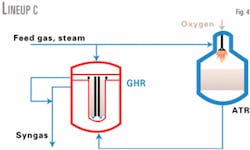
Lineup C (Fig. 4) has a similar parallel configuration to Lineup B. The difference is that that the two reformed gas streams do not mix in the GHR. Syngas from the GHR catalyst is cooled separately in bayonet tubes that are within the annular catalyst beds in each outer tube.
The relative effect that these lineups have on the FT production process, as well as the comparative size of GHR needed for each duty, can be simulated for different reforming conditions.
The GHR catalyst exit temperature is a key process parameter. It determines the reduction in oxygen flow compared to a standalone ATR. It also influences the GHR's size, because the GHR is simply a heat exchanger with catalyst in the tubes-the higher the tube exit temperature, the lower the temperature differential driving heat transfer.
Due to less gas passing through the catalyst, Lineups B and C can have a lower pressure drop than Lineup A. Therefore, they must have a higher GHR exit catalyst temperature to keep the levels of unreacted methane acceptably low.
Table 3 and Fig. 5 show a comparison between these schemes. In process terms, Lineups B and C are identical. An important differentiator between these lineups is the total concentration of CH4, CO2, N2, and Ar in the syngas product. These gases are inert in FT synthesis-the higher the inerts level, the worse the FT gas quality.
Even with GHR catalyst exit temperatures up to 1,650° F., the total inerts level is still much higher in the parallel lineups than the series configuration. This results in lower process efficiency and an increased requirement for syngas and oxygen production.
Lineups B and C show the same upward trend in heat transfer area (HTA) with increasing catalyst (or tube) exit temperature. If the tube exit temperature is 1,560° F. (process performance not competitive with lineup A), the equipment size will tend to be the same or larger. At 1,650° F., there is still a 5% efficiency penalty, and the HTA requirements are more than twice those of the series arrangement.
Altering other variables-such as splitting steam between the GHR and ATR, or considering a GHR in combination with a non-catalytic partial oxidation reactor rather than an ATR-can result in minor improvements compared to the parallel schemes, but they do not affect the overall results.
A series configuration is both smaller and more efficient than a parallel GHR and ATR combination.
GHR development
Synetix first developed the leading concept ammonia (LCA) process, which incorporated GHR, in the 1980s. LCA was applied to ammonia production with plants being commissioned near Bristol, UK, in 1988 and later at Yazoo City, Miss., in 1998.
In the early 1990s Synetix adapted the technology for methanol production as the leading concept methanol (LCM) process. A demonstration methanol unit has been operating near Melbourne, Australia, since 1994.
In the latest extension, we have applied GHR technology to the syngas production for FT-based GTL plants.
Advanced GHR
The early applications of GHR technology were successful. Technology development continued during the 1990s when it became apparent that there were some features of the first-generation GHR that could be improved.
For example, catalyst loading was more difficult and time consuming than in a conventional steam reformer. Some design aspects of the bayonet tube units were complex, which added to the unit cost and limited the maximum size.
By 1998, Synetix developed an improved and simplified design of GHR known as the advanced gas heated reformer (AGHR). Synetix installed a demonstration unit in the methanol plant now operated by Coogee Energy Pty Ltd., Melbourne, to test the novel features of the new design.
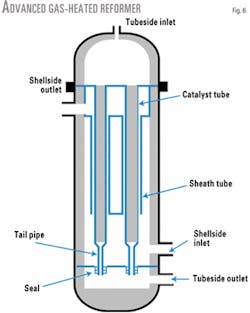
The AGHR (Fig. 6) has a simplified internal design with a seal system that accommodates tube expansion without the need to use bayonet tubes.
These changes simplify the design and fabrication and result in a substantial reduction in unit cost. Catalyst loading and removal is also much easier and performed the same way as a conventional steam reformer using traditional sock loading or an advanced catalyst loading system.
Detailed engineering studies show that the AGHR can be scaled up to a capacity of 25,000-30,000 b/d in a single train. The Coogee AGHR has been in operation for more than 3 years and has proved the unit's new design features.
Metal dusting corrosion
AGHR plants for methanol and ammonia production have operated for many years, and the materials of construction as well as the process and mechanical design are fully established for these applications.
Using an AGHR for GTL offers a significant improvement in thermal efficiency. Even more efficiency gains and capital cost benefits result from more severe reforming conditions, specifically a lower SR. The optimum SR is normally between 0.6 and 1.0, the exact optimum of which depends on the individual project.
No materials of construction have been commercially demonstrated to operate successfully at the lower SR conditions required for the optimum use of GHR technology in GTL plants. Experience shows that, while laboratory and other small-scale testing may provide some limited initial screening of materials, it cannot provide reliable data on the performance of an alloy under plant conditions.
Materials demonstration unit
The MDU has two GHRs with full-size tubes, each coupled to a secondary reformer and associated equipment. The two trains operate at different conditions to demonstrate metallurgy suitable for GTL and enhanced methanol process conditions.
In an experimental program initially lasting 18 months, the MDU will test several different alloys simultaneously with conditions closely replicating those of a full-scale commercial unit. The unit will provide long-term data under commercial plant conditions.
References
- Chang, T., "New JV markets one-stop GTL package," OGJ, Dec. 18, 2000, p. 49.
- Steynberg, A.P., Vogel, A.P., Price, J.G., and Nel, H.G., "Technology targets for gas to liquids applications," presented at the AIChE Spring meeting, Apr. 22-26, 2001, Houston.
The authors
Jim Abbott is a GTL technology specialist for Synetix, Bill ingham, UK, in which he focuses on delivering the application of syngas technology to GTL. Recently, he was engaged in process development of Synetix's refinery business. Abbott joined ICI 22 years ago and joined the Katalco group in 1987. He has worked for 8 years in the methanol business, in which he played a key role in demonstrating the Synetix leading concept methanol technology. He has a degree from Cambridge University, UK.
Bernard J. Crewdson is a business development manager for Synetix's GTL and fuel cells businesses. He joined joined ICI in 1975 and held a number of posts in process engineering and production management before joining Synetix (formerly Katalco) in 1987. Crewdson also spent 10 years in catalyst sales and marketing. He graduated from Cambridge University with a degree in chemical engineering.