Unit converts H2S to raw material for lube-additive production
Lubrizol, Le Harve, France, converts hydrogen sulfide (H2S), a byproduct of its lube-oil additive production, into sulfur, a raw material for additive manufacture.
Without this conversion system, the H2S would be a pollutant.
The system, called Lo-Cat, requires a large turndown because the Lubrizol plant operates in batch mode.
Turndown, a measure of a system's flexibility, is the ratio of the maximum design gas flow rate to the minimum expected gas flow rate.
For example, a turndown ratio of 10 means that the design gas flow rate is 10 times greater than the minimum expected gas flow rate.
Both flow rate and H2S concentration parameters in the Lubrizol plant vary from 0 to 100%. Few other H2S removal processes can tolerate such a large turndown.
Sulfur produced from the Lo-Cat unit is recycled back as raw material to the batch processes, which produce the sour gas. Unique design features in the Lo-Cat unit produce high quality sulfur. The use of the Lo-Cat sulfur has resulted in no deterioration of the additive.
By recycling the sulfur, Lubrizol has reduced its purchases of raw sulfur from other sources, saving about $35/tonne of sulfur. Fig. 1 is a photo of the Lubrizol plant.
Process description
In May 1994, Lubrizol purchased a Lo-Cat II Hydrogen Sulfide Oxidation System from USFilter's Gas Technology Products Group. The facility in Le Harve produces a proprietary lube-oil additive.
The Lo-Cat unit has two design features, unique for this installation.
First, the unit is capable of a large, repetitive turndown requirement. The batch nature of the production of the lube-oil additive uses elemental sulfur as a reactant and produces H2S as a byproduct.
The batch reaction cycle has an 8-hr period during which the H2S concentration in the effluent gas from the batch reactor varies from 0% to 100%. This variation in H2S concentration requires a large, repetitive turndown from the Lo-Cat unit.
Second, Lo-Cat produces sulfur of sufficient quality not to degrade the product. Elemental sulfur produced in the unit is recycled back to the front end of the process as feed to the batch reactors.
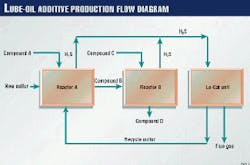
Lubrizol's Le Harve facility produces its lube-oil additive in two separate batch reactions (Fig. 2). The additive's batch reactions are proprietary.
Generally, the reactions can be represented as follows:
First batch reaction
New sulfur + recycled sulfur + Compound A > Compound B + H2S (gas)
Second batch reaction
Compound B + Compound C > Compound D + H2S (gas).
Compound D is the desired lube oil additive.
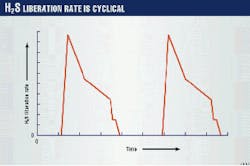
The H2S generated in both batch reactions is sent to the Lo-Cat unit; approximately 60% of the total H2S sent to the Lo-Cat unit was generated in the first batch reaction. Fig. 3 illustrates the variation of H2S during the batch cycle. For every two batches, the Lo-Cat unit produces and recycles about 3,000 kg of sulfur.
Lo-Cat II process
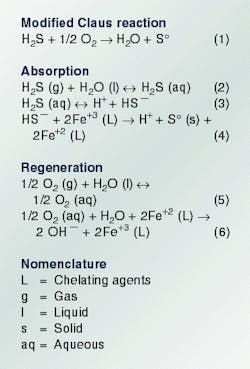
The Lo-Cat II process is an isothermal, low operating-cost method for carrying out the modified Claus reaction shown in the above box.
The reaction is carried out in an aqueous solution. The solution contains a metal ion capable of removing electrons (negative charges) from a sulfide ion (S=) to form elemental sulfur. The ion, in turn, transfers the electrons to oxygen (O2) in the regeneration process.
Although many metals can perform these functions, Lo-Cat II uses iron because it is inexpensive and nontoxic.
The basic reactions of the Lo-Cat II process are absorption and regeneration. Reactions for these two processes are in the accompanying box. The sum of all these reactions is the reaction in Equation 1.
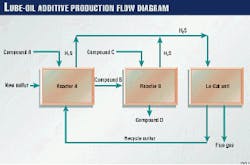
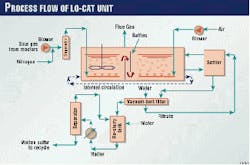
Fig. 4 shows a process flow diagram of the Lo-Cat II system that was furnished to Lubrizol-France.
After compressing the sour gas from the batch reactors, the plant sends the gas through an inlet knockout pot to remove any entrained liquid droplets. The process then sparges the sour gas in regenerated Lo-Cat solution in the absorber sections of the autocirculation vessel.
Rotary-lobe air blowers supply air, which is sparged through the reduced Lo-Cat solution in the oxidizer sections of the autocirculation vessel. The differences between the aerated densities in the absorber and oxidizer create an "air lift."
This lift circulates the iron catalyst throughout the autocirculation vessel. In this way, both the H2S oxidation to sulfur and the regeneration of the iron catalyst occur in one vessel.
The process pumps a small slipstream of the solution to a cone-bottomed, sulfur settler vessel. In this vessel, the sulfur is concentrated into slurry of approximately 10 wt % sulfur.
From the sulfur settler, progressive cavity pumps deliver the sulfur slurry to a vacuum belt filter, which produces a sulfur cake of approximately 60 wt %. The filter cake is water washed, and the filtrate (catalyst solution) and the wash water are pumped back to the autocirculation vessel.
The filter cake falls off the belt filter into a reslurry tank where water is added to produce a 10 wt % sulfur slurry. Again, a progressive cavity pump directs the sulfur slurry from the reslurry tank into the melter, a patented, vertical, shell-and-tube exchanger.
From the melter, the molten sulfur flows to a separator where the molten sulfur settles to the bottom and water rises to the top. The molten sulfur is recycled back to batch reactors.
There were three main areas of concern in the design phase:
- H2S absorption as the concentration approached 100% H2S.
- Treating instantaneous H2S loads of 10 tonnes/day in equipment sized to handle an average H2S load of 3 tonnes/day.
- Production of high-quality sulfur suitable for recycle.
The rate at which H2S absorbs into aqueous solutions such as are employed in a Lo-Cat unit depends on the partial pressure of H2S in the gas stream, the degree of contact between the gas and the aqueous solution, and the pH of the solution.
In a Lo-Cat autocirculation unit, the absorber portion of the vessel is a simple column through which the sour gas is sparged through a column of Lo-Cat solution. A high degree of contact between the gas and the liquid is maintained by developing small gas bubbles with large relative surface areas.
This is done by using high-velocity gas nozzles and by maintaining high gas velocities through the liquid in order to create shear on the surface of the gas bubbles.
When the H2S concentration approaches 100%, the amount of the gas and, consequently, the gas velocity through the liquid decrease rapidly as the bubbles rise through the liquid, and the H2S gets absorbed into the solution.
This reduction in gas velocity and turbulence reduces the shear on the surface of the bubbles, and the absorption rate and the removal efficiency of H2S decreases.
To counteract the reduction in gas velocity as the bubbles rise through the solution, two design changes were incorporated to increase shear so that the outlet H2S specifications were met:
First, a nitrogen-gas stream was mixed with the sour gas streams from the reactors just upstream of the Lo-Cat unit. Since nitrogen is virtually insoluble in the solution, the velocity of the gas bubbles would be maintained at a certain minimum value as the H2S gets absorbed.
The second design change incorporated a mixer in the absorber portion of the vessel. The mixer provides the shear to the solution, which is lost because of the lower bubble velocity.
As illustrated in Fig. 3, the rate of H2S entering the Lo-Cat unit is cyclic. The maximum instantaneous H2S rate is equivalent to 10 tonnes/day of sulfur; however, the average daily H2S loading is equivalent to only 3 tonnes/day.
If the autocirculation vessel is sized for less than the maximum sulfur capacity (10 tonnes/day), the iron could become over-reduced and precipitate as FeS. This would increase operating cost and reduce removal efficiency during the periods of over-reduction.
For this reason, the absorber and oxidizer sections of the autocirculation vessel are sized for 10 tonnes/day of sulfur. Since the autocirculation vessel is now oversized relative to the average sulfur loading, however, the capacitance of the Lo-Cat solution to hold solid, elemental sulfur is large. Consequently, the sulfur-handling portion of the plant is designed for the average sulfur loading.
The most unique feature of this Lo-Cat unit is that all of the sulfur produced in the unit is reused as a raw material in the upstream process. Consequently, the sulfur is 98+% pure and contains no more than 200 ppm (wt) ash. The unit achieves these sulfur specifications.
To meet this purity, the sulfur produced in the unit is filtered and water-washed on a vacuum belt filter. The filtrate is returned to the Lo-Cat unit, and the 60 wt % sulfur cake from the filter is reslurried with water to produce 10 wt % slurry, which is sent to the melter system.
The quality of the molten sulfur from the sulfur separator far exceeds the specified purity and use of the Lo-Cat sulfur has not resulted in any deterioration of the product.
The Authors
Rosalind Cantrall is sales and marketing manager at USFilter/Gas Technology Products. Previously, she worked at UOP as a process engineer. Cantrall holds a BS in chemical engineering from the Georgia Institute of Technology, Atlanta.
Gary Nagl is vice-president and general manager of USFilter/Gas Technology Products. He manages the operations, sales, marketing, and research and development efforts for USFilter's sulfur-recovery business.
Prior to joining USFilter, Nagl held similar positions with Wheelabrator Technologies, ARI Technologies, and UOP. He holds a BS in chemical engineering from the University of Illinois, Urbana.