Water content affects low pressure, acid-gas metering
Failure to consider the water content at metering conditions can lead to a significant error in the calculation of the actual acid-gas flow rate.
Acid gas is a mixture of mainly H2S and CO2 that are separated from a sweetening solution in the reflux drum, usually a horizontal separator, in a sour-gas sweetening process. Also present in the mixture are some hydrocarbons, generally less than a 2% concentration. Water vapor, however, fully saturates the acid-gas mixture at the pressure and temperature in the reflux drum.
Most operators generally meter the acid-gas mixture before it flows to either a sulfur-recovery unit, to flare, or to compression and injection into subsurface strata. Because a laboratory always determines the acid-gas composition on a dry basis, operators sometimes ignore the water content in the gas at the meter.
Meters
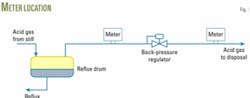
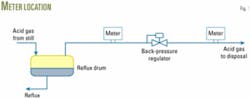
Fig. 1 illustrates the choices for installing a meter for measuring the acid-gas flow exiting the reflux drum. The two possible locations for low-pressure measurement are either upstream or downstream of the backpressure regulator of the reflux drum.
While most operators choose an orifice meter, other meters that could be installed include a vortex-shedding meter, thermal mass-flow meter, annubar meter, and venturi tube meter.
Water-vapor estimate
The water-vapor content in the acid gas depends on the pressure and temperature in the reflux drum. Because the laboratory report provides the acid-gas analysis on a dry basis, an operator must calculate the water-vapor content in the acid gas at reflux-drum conditions. The use of Raoult’s Law at low pressure can provide an estimate of the vapor content.
The mole fraction of water vapor in the acid gas equals the ratio of the vapor pressure of water at the reflux-drum temperature to the absolute pressure in the reflux drum. Thermodynamic tables can supply the vapor pressure of water, or one can estimate it from Equation 1 (see equation box).1
Equations 2 and 3 convert Equation 1 for calculating vapor pressure directly in kPa or psia.
The mole fraction of water vapor in the acid gas is then the vapor pressure of water as determined by Equations 2 or 3, divided by the absolute pressure in the reflux drum, in the respective units.
A meter downstream of the backpressure regulator would have a meter temperature and pressure lower than in the reflux drum. In any case, one has to determine the water-vapor content on the basis of the conditions in the reflux drum. Thus it is important always to record the reflux-drum pressure for this purpose, in addition to the meter readings of pressure and temperature for the flow-rate calculation.
For meters downstream of the regulator valve, the reflux-drum temperature will drop slightly due to the reduced acid-gas pressure. Equations 4 and 5 can estimate the temperature in the reflux drum if the acid gas is not heated or cooled
Example calculation
An example calculation shows the effect of ignoring the water content in low pressure, acid-gas metering with an orifice meter, although the same holds true also for other meters.
The calculation assumes a meter size and meter readings as shown in Table 1.
The procedure for proper acid-gas flow metering at or below reflux-drum pressure should therefore include the conversion of the acid-gas analysis from a dry basis to a wet basis. All calculations of fluid properties should then be based on the properties of the wet fluid stream. Table 2 illustrates this for an assumed dry acid-gas analysis and the reflux-drum pressure and temperature, as shown in Table 1.
The H2S and CO2 content can vary greatly, but the hydrocarbon content in a well-run sweetening system is generally less than 2% hydrocarbon with the acid-gas components.
From Equation 2, one obtains a vapor pressure of water of 9.5825 kPa absolute at the reflux-drum conditions of pressure and temperature (Table 1). The mole fraction of water in the acid gas is 9.5840/(80 + 95) or 0.05477
The gas relative density is based on the molar mass values in Fig. 23-2 of the latest GPA/GPSA Engineering Data Book,2 and the compressibility factors are based on the Wichert-Aziz method of compressibility-factor calculation, which was adjusted for water-vapor content.3 The compressibility factors obtained from the AGA Report No. 8 method are virtually identical to those listed previously.4
Using the data from Tables 1 and 2, one can use the orifice-meter calculation routine based on the AGA Report No. 3 Standard, 1985 Edition. These calculations can easily be checked manually.
Table 3 provides the results of these calculations.
Table 3 also shows the results of additional calculations with AGA Report No. 3, 1992 Edition with AGA Report No. 8 for compressibility factors and the same molar mass for the components as in the previous method.
Temperature in the reflux drum greatly influences the discrepancy between dry and wet-based calculations because the vapor pressure of water is higher at higher temperatures.
Results
Column 2 in Table 3 shows the flow rate calculated on the basis of the dry acid-gas composition, as shown in a laboratory analysis. The wet flow rate in Column 3 includes the water-vapor content in the acid-gas composition.
One has to correct the wet rate to the equivalent dry flow rate by multiplying the wet rate by (1-Mole fraction of H2O).
In Column 4, the actual dry flow rate is 4.25% (or 4.29%) less than the dry rate obtained by not including the water vapor in the flow stream. The column shows similar differences in the sulfur content when the calculations ignore the water content in the acid gas. ✦
References
1. Dean, J.A. (editor), Lange’s Handbook of Chemistry, 11th Edition, New York: McGraw-Hill Book Co., 1973; pp 10-45.
2. Engineering Data Book, 12th Edition, Tulsa: Gas Processors Association/Gas Processors Suppliers Association, 2004; p. 23-2.
3. Wichert, E., and Aziz, K., “Calculate Z’s for sour gases,” Hydrocarbon Processing, Vol. 51, No. 5, May 1972, pp. 119-22.
4. Starling, K.E., and Savidge, J.L., Compressibility Factors of Natural Gas and Other Related Hydrocarbon Gases, AGA Report No. 8, 2nd Edition, Arlington, Va.: American Gas Association, 1992.
The author
Edward Wichert is an independent oil and gas industry consultant in Calgary. He has experience at the technical level as well as in management in drilling, oil and gas production and processing, reservoir engineering, and economic analysis. His research interests are mainly related to sour gas. Wichert holds a BSc in petroleum engineering and a masters in chemical engineering.