Formosa refinery improves sweetening with amine blends
Chang-Chen Liu
National Cheng Kung University, Taiwan
Formosa Petrochemical Corp.
Jung-Hua Wu
National Cheng Kung University
Taiwan
Formosa Petrochemical Corp. (FPCC) reduced the mass flow rate to regeneration and increased the absorption and regeneration capacity at its amine treating regeneration system after replacing diethanolamine (DEA) with methyldiethanolamine (MDEA)/DEA blends on line.
The required MDEA/DEA blending formulation was at a ratio 1.647, which was based upon the CO2 specification of the downstream residue catalytic cracking unit (RCC). Utilizing the highly efficient MDEA/DEA blends reduced the amine circulation rate and mass flow rate to regeneration by around 18%.
This decrease resulted in steam consumption being reduced by 24% and the gas processing capacity being increased by 12% by improving the efficiency and introducing new expansion absorbers on line.
Introduction
Feed gases to the absorbers derive from the following upstream units in FPCC's refinery, Taiwan:
1. Low-pressure feed gases produced from catalyst cracking, delayed coking, or the other thermal cracking processes.
2. Recycle gases from hydrotreating, hydrocracking processes, and process stabilizers.
3. LPG streams.
4. Sulfur-plant tail gas.
Various feed gases, the recycle gas from hydroprocessing unit at 40-80 kg/sq cm (g) pressure, the hydrocracking unit at 150-210 kg/sq cm (g), or about 2,204~3,086 psig pressure, and all the other units at 13~23 kg/sq cm (g) pressure are introduced at the bottoms of the individual absorbers, which is packed or trayed, and contacted counter currently with amine solution absorbing the acid gas in the amine.
Rich-amine solution leaving the absorbers flows to the regenerator, contacting stripping steam generated by the reboiler. The hot lean amine from the reboiler is then circulated back to the absorber.
It is a simple absorption reaction, as shown in the first reaction in the accompanying box. H2S reacts instantaneously with the primary, secondary, or tertiary amine via a direct proton transfer reaction to form amine hydrosulfide.
The reaction between the amine and CO2 is a bit more complex because CO2 absorption can occur via two different reaction mechanisms. When dissolved in water, CO2 hydrolyzes to form carbonic acid, which in turn, slowly dissociates to bicarbonate. The bicarbonate then undertakes an acid-based reaction with the amine to yield the overall reaction, shown in the second set of reactions in the accompanying box.
With the H2S and CO2, reactions shown in the third set will occur simultaneously.
In a refinery, methanolamine (MEA), DEA, and MDEA are generally used to remove H2S in the amine unit.
MEA, a primary amine, has higher reaction heat with acid gas and comes up with higher stripping steam consumption. If carbonyl sulfide (COS) is present in feed gas, MEA will irreversibly react with it and carbon disulfide (CS2) to form a urea derivative resulting in degradation of the solvent.
Using MEA in low H2S partial-pressure service (13~22 kg/sq cm g) leads to low acid-gas loading, which is around 0.22 mole H2S/mole of MEA, increasing the cycle rate of amine. MEA is the strongest base of alkanolamines; corrosion and foaming have been the major difficulties with its use. Therefore a 15~20% solution in water is most often used for sweetening.
DEA is a secondary amine and was applied in sweetening gas streams of FPCC's refinery. As DEA will react regeneratively with COS and CS2, which usually is formed in cracking conditions, it was selected for this service. DEA has lower heat of reaction than MEA. The disadvantage is DEA will seldom reduce the H2S content to a low ppm level.
For a hydrotreating recycle gas service in high-pressure conditions (40~180 kg/sq cm g), the acid gas loading of 25% DEA solution can reach greater than 0.42 mole H2S/mole of DEA. Typical DEA concentrations are around 20~30wt %.
MDEA, a tertiary amine, does not have hydrogen attached to the nitrogen; the CO2 absorption can only occur after CO2 dissolves in water to form a bicarbonate ion. Therefore, in the presence of CO2 it selectively absorbs H2S from gas while leaving a large amount of CO2 in the gas.
MDEA can be used in higher amine solution concentrations. It has a number of properties that make it desirable for broader application over primary and secondary amines including higher acid-gas loading with fewer corrosion problems, lower reaction heat leading to lower stripping steam consumption, higher resistance to degradation, and lower vapor pressure leading with lower amine losses.1
While stronger acids were present and reacted with amine, heat stable salts (HSS) were formed. HSS will cause higher make-up amine solvent costs, increase the corrosion rate of the system, and block the pipeline and column slot. So the system should use a caustic (NaOH) solvent to do base treatment to regenerate with distillation or recover with ion exchange reclamation, electrodialysis reclamation, or reverse-osmosis reclamation to avoid destroying the amine because of high temperature.
Selection of amine solvent and cycle concentration depend on facility cost, operation and maintenance costs, and some special requirement of the system such as removing H2S selectively.
In addition, the absorption of sour LPG streams is a liquid-liquid treatment mechanism; it is better to use low-viscosity amine for preventing entrainment loss and solubility loss. The purpose of treating tail gas from a sulfur-recovery plant (SRU) is to reduce SOx being discharged from a thermal oxidizer, so that the SRU tail gas generally uses formulated MDEA to absorb H2S selectively. Designed cycle concentration is around 45 wt % and H2S concentration of the tail-gas should be lower than 10 ppm (vol).2
Furthermore, in the presence of CO2, MDEA selectively absorbs H2S from gas while leaving a large amount of CO2. The selectivity results from the inability of tertiary amines to form a carbamate with CO2; MDEA has higher H2S-removal capacity compared with DEA. DEA degradation with CO2 to form by-products has been demonstrated.
On the other hand, MDEA/DEA blends have been successfully used in a wide variety of gas-treating applications, and plant data show corrosion and DEA degradation of MDEA/DEA blends are much less of a problem.3 Therefore, MDEA/DEA is an efficient selection when feed gas contains CO2, as it can remove CO2 down to the required specification of downstream and selectively remove H2S at the same time.4
Meanwhile, the reason to remove H2S is mainly for security (H2S is poisonous), avoiding pipeline corrosion and pollution caused by SO2 because of burning the tail gas. The purpose to remove CO2 is to meet pipeline anti-corrosion requirements or to meet specifications downstream, for example, to avoid blocking the cold box cooler because of freezing of the CO2 and to prevent catalyst poisoning.
Table 1 shows property comparisons among DEA, MDEA, and MDEA/DEA blends.
As MDEA has all the advantages described above, replacing amine with MDEA or MDEA/DEA blends in feed gas with CO2 can reduce regeneration-steam consumption and increase gas sweetening.5 6
Based on planning to gain MDEA's advantages along with considering a certain amount of CO2 contained in the feed gases of amine absorbers in FPCC refinery's, this article discusses the use of high-efficient MDEA/DEA blends in the amine system of FPCC's refinery.
System optimization
FPCC's refinery has five identical amine regenerators in centralized amine-regeneration system service for 15 amine absorbers.
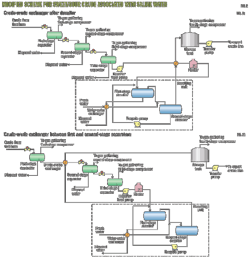
Before conversion, the centralized amine-regeneration units (Fig. 1) were designed to process 2,597 cu m/hr of rich amine in total returned from all the absorbers with rich-amine loading averaging around 0.41 mole/mole. Rich amine from an amine absorber in each process unit was mixed to be sent to amine regeneration. Regenerated lean amine is returned to each amine absorber of the process unit for circulation. Resid catalytic cracking units in FPCC's refinery have CO2 in the feed gas. An RCC downstream unit has a low-temperature treating process that limits CO2 concentration. RCC-treated sweet gas should be reduced to less than 500 ppm (vol) while removing almost all the H2S. There should be bypass caustic scrubber operation to protect downstream low-temperature treating treatment, or a downstream process unit should use a caustic scrubber to remove excess CO2 in order to meet the CO2 spec of less than 500 ppm (vol).
FPCC's refinery was planning to increase refinery capacity to 120%. In addition, eight new absorbers from new hydrocracking, hydrotreating, and sweetening process expansion projects were planned to be built for producing more valuable oil products, and the existing hydrogen-desulfurization unit had to be reconstructed to perform deep desulfurization to meet market specification for gasoline products below 10 ppm of sulfur content in 2010.
An amine-treating process was designed into two systems: low pressure (refinery) system and high pressure (hydrotreating) system. Both systems used 25% DEA for H2S and CO2 absorption. After expansion and deep desulfurization of the process units, the amine cycle rate had surpassed the original design by 18%. That caused bottlenecking in some H2S absorbers.
Besides the increased cost of steam, power, capital investment in additional amine units is increasing the cost of operations, which forced this refinery to search for an improved amine solvent to increase the treating capacity at lower operating cost rather than installing new amine units at additional capital investment. And the refinery had planned to replace the DEA amine solvent with an MDEA/DEA blend.
The optimum solution concentration and the blending ratio of MDEA to DEA depend on the acid-gas concentration in the feed, absorber pressure, and temperature along with sweet-gas requirement. Several simulations at various concentrations and MDEA/DEA blends used the process simulation program TSWEET/ProMax to predict the optimized operation conditions. The purpose of the simulation was to get the best MDEA/DEA blend at optimized operation conditions to minimize amine circulation rate and operation duties of rich-lean heat exchanger, regenerator condensers, regenerator reboilers, and lean-amine coolers and to meet the CO2 specification in treated gas.
Simulation results helped management decide to go for amine conversion. The selected MDEA/DEA blend is 45 wt % MDEA/DEA blend with ratio of MDEA to DEA at 1.647. This MDEA/DEA blend can treat H2S and CO2 content to the desired levels. Table 2 shows the simulation results for the adsorbing properties of a 45 wt % MDEA/DEA blended amine system. Due to the new 45 wt % MDEA/DEA blend, the solvent circulation rate was reduced by 598 cu m/hr totally (29.5% less than 25 wt % DEA), and total steam consumption for regeneration reduced by about 73 tonnes/hr (28.4% less than 25 wt % DEA). Simulation results indicated the higher sweetening capacity in absorbers at lower amine circulation rate.
Planning, implementation
There are two options for converting the amine treating system from DEA to MDEA/DEA blends. One is to shut down the unit and clean and reload it. The other is to add formulated MDEA in several phases until the target amine solution concentration and blending ratio are reached without having to shut down the amine unit.
Before conversion planning, the systems were surveyed. A corrosion measurement report, amine solvent analysis report, and inspection report indicated that all the equipment was in good condition and there was no need to shut down the amine unit and drain the system of DEA solvent.
The lean DEA sample analysis result reported slightly higher HSS in the lean amine than the amine solvent supplier recommended. But there was no evidence of corrosion in the system. The percentage of HSS can be brought down by the addition of MDEA amine solvent to obtain the MDEA/DEA blend; furthermore, shutting down the amine unit was not possible.
FPCC decided to go for Option 2 of MDEA/DEA blend on line conversion and monitored amine system performance continuously.
The conversion was performed with two targets in view: CO2 specification in sweet gas and correct MDEA/DEA blend ratio. The first target was achieved by continuous measurement of CO2 content in the RCC sweet gas, and the second target was also obtained by composition analysis of MDEA and DEA in the amine blend.
The conversion was from third-quarter 2008 to third-quarter 2009. Total conversion implementation was planned for several steps over 3 months for both amine systems (refinery and hydrotreating). The well chosen MDEA/DEA ratio was 1.647, and the final MDEA/DEA blend's composition was planned to be reached in 3 months.
The existing amine regeneration system has seven amine tanks. Each system conversion kept one tank (labeled A) for fresh MDEA and put two tanks (labeled B and C) for lean- amine operation. Following are the procedures for on line blending for each amine system:
1. Empty Tank A and C and clean with demineralized water after isolating from the amine system.
2. Fill Tank A with fresh 100 wt % formulated MDEA.
3. Bring down the amine level to 10% in Tank B by diverting 25% DEA solution to Tank C level not more than 40%. Keep the amine unit operation stable with 10% level in amine storage Tank B.
4. Charge fresh 100 wt % MDEA to Tank B from Tank A continuously for mixing with 25% DEA and obtain the required MDEA/DEA blend both in concentration and MDEA/DEA ratio on line.
5. Pump MDEA/DEA blends to all absorbers. Maintain all operational parameters the same as during DEA operation mode for a few days. Check the MDEA/DEA blend concentration and ratio until the required conditions are reached.
6. Optimize the amine flow rate to all service absorbers slowly and then optimize the steam flow rate to the regenerator reboilers as calculated by ProMax simulation.
7. Check the treated-gas specification for H2S (below 10 ppm) and CO2 (below 1,900 ppm) in the RCC absorber.
During the conversion runs, the concentrations of MDEA/DEA blends were measured and monitored each day.
The lean-amine demand rate of each absorber was slowly reduced right after the blended amine strength was 45 wt %, and the treated-gas qualities of each absorber were being measured. These measurements and monitoring were continuously done and recorded until 45 wt % total blended amine strength and targeted lean-amine demand rates for each absorber were reached.
Conversion results
During the conversion, the amine circulation rates were decreased step by step to the targeted rates of each absorber and the sweet gas was analyzed at the same time. The process parameters were recorded and the performance of equipment was carefully controlled every hour.
Conversion helped to increase the total sweetening capacity, and the amount of H2S in each vessel's treated gas was lower than before. The results showed that MDEA/DEA blends require lower circulation, so that the regeneration steam also decreased immediately after the conversion day and one lean-amine pump was also stopped.
• CO2/H2S content in RCC treated gas. The RCC unit was built in 2000 and had been revamped to 130% in 2005 without revamping the tail-gas absorber. The absorption rate was calculated separately using 2 in. and 3 in. weir heights in the ProMax Simulation, and the results reported were 500 ppm (vol) for the 3-in. weir height and 1,900 ppm (vol) CO2 for the 2-in. weir height. Current weir height is 2 in. Simulation with 3-in. weir height (retrofit from 2 in.) was for an expected revamping in the near future.
During conversion the trays were not retrofitted and the CO2 gas product specification was targeted to meet the ProMax simulation result, 1,900 ppm (vol). The conversion of this unit (refinery system) took place in July 2008. Before conversion, CO2 content in the treated gas was 5,190 ppm (vol) at 46.6º C. lean-amine temperature. Since maximum lean-amine rate was 70 cu m/hr (limited by the capacity of absorber), the target value calculated by ProMax simulation could not be reached.
The CO2 reaction with MDEA is kinetically controlled; the plant had increased the lean MDEA/DEA blends temperature to 54º C. from 47º C. to increase the reaction rate, and the lean-amine rate of the RCC absorber had also increased to 70 cu m/hr from 62 cu m/hr to increase the CO2 pickup during conversion.
These operational parameters helped lower the CO2 content in treated gas to 2,510 ppm (vol) from 5,190 ppm (vol; Table 3), which is close to the result of the ProMax simulation with 2-in. weir height. With the higher content, the RCC absorber's treated gas still has to bypass the caustic-wash system to protect downstream process.
On the other hand, this CO2 content was still lower than the piping specification and a better quality of acid gas was drawn into the sulfur-recovery plant. The H2S content in the RCC sweet gas was improved and lowered to 1 ppm (vol) from 10 ppm (vol), and the total conversion was performed smoothly and efficiently.
The second-phase conversion was to remove H2S. This phase was implemented in July 2009 after a period of stable monitoring of the refinery amine system's operation and waiting for amine preparation. The amount of H2S in the treated gas was less than 1 ppm (vol) which was confirmed by laboratory. The H2S removal improved by around 4.5% in kg mole, and it was expected that H2S content in the acid gases could be increased.
• Circulation rate and energy saving. The conversion of MDEA/DEA blends demonstrated that high solution concentration and high acid loading of MDEA/DEA blends result in lower circulation rates for any given absorbers. The total reduction in circulation rates was 450 cu m/hr, which was 75% of simulation result. Table 4 shows the changes in circulation rate and H2S content in the outlet gases of every absorber after total conversion in October 2009.
According to the simulation result, the actual rate of the RCC had to increase by 65 cu m/hr, but it only increased by 12 cu m/hr, limited by the capacity of absorber, and resulted in high CO2 content in the RCC treated gas, as mentioned previously. For the remaining absorbers, the H2S content was lower than simulated, providing the opportunity to lower circulation rates in future.
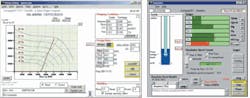
Fig. 2 also shows the trend of outlet H2S content and circulation rate for the units with decreasing circulation rate during the conversions. The outlet H2S content in vacuum gas oil (VGO), hydrodesulfurization (HDS), and resid desulfurization (RDS) absorbers included the H2S amount bypassed from the feed gas of the absorbers (5-6% flow bypassed). These bypass streams are for keeping the treated stream contents with sufficient H2S, while circulating to reactor for hydrotreating reactions.
Average experimental analysis for H2S contents in the absorber top outlets were less than 1 ppm (vol). During the conversion, the bypass flow of the VGO absorber had slightly increased to moderate the hydrogenation reaction, and this contributed 400 ppm (vol) H2S content in combined flow as shown in Fig. 2a.
Comparing the H2S content in each absorber outlet, converting to MDEA/DEA blends resulted in lower outlet H2S content and required circulation rate, and the latter decreased the steam consumption.
Steam consumption has decreased from the conversion because blended amine requires a much lower circulation rate and desorbing energy than DEA. Steam used to strip the amine of H2S is controlled on a steam/solution ratio. Lowering the circulation rate decreases steam consumption.
As shown in Table 5, the actual amine circulation was lowered to 1,649 cu m/hr after the hydrotreating system was converted in October 2009. During conversion, the new expansion absorbers were lined up and the system drew 425 cu m/hr amine solution saved from the amine conversion plan and made the total steam saving 61 tonnes/hr at 0.10 steam/solution ratio after total conversion.
Corrosion consideration
The plant had done the sample analysis before the conversion and the result shows that HSS were at a slightly higher level than the recommended operation conditions but still kept in acceptable range from corrosion. Total anion analyzed was around 0.73% including organic and inorganic.
HSS formation and corrosion rate are lower in the case of MDEA-based solvent,7 and partial draining with fresh-feed make up will keep the HSS in a lower range.8 9 The amine systems were stabilized during that time. After system evaluation, the plant decided to mix fresh MDEA formulated into 25% DEA solution and monitor the system continuously.
The following actions were taken during and after the conversion procedures to prevent amine blends from degrading and equipment from corroding; lean-amine solutions were analyzed every 3 months:
1. Changed the filter element at least to 10 μ to remove the suspended iron particles to control corrosion and erosion.
2. Provided cold condensate to the top of the inlet-gas separators and continuously sprayed to wash away the contaminants in sponge gas.
3. Drained reflux from regenerator condenser to a sewer for a period of time and used cold condensate at the same volume to maintain the water balance in the regenerator.
4. Bypassed a higher rate of lean amine to the activated carbon filter for further cleaning up.
5. Checked and monitored the corrosiveness of the lean and rich amine at the following locations:
a. Lean amine at storage tank.
b. Lean amine after lean-amine cooler.
c. Hot lean amine at the regenerator bottom outlet.
d. Rich amine at the bottom of the absorbers.
e. Rich amine at the inlet of the regenerators.
The use of blended amine solvents for gas sweetening had been investigated using a simulation program; conversion was performed by adding 100% concentrated MDEA during operation.
The total circulation rates of the central gas-sweetening systems of FPCC's refinery were decreased by 18% as a result of switching solvents from DEA and MDEA/DEA blends. This decrease resulted in 24% reduction of steam consumption. The gas processing capacity was increased 12% by improving the efficiency and introducing new expansion absorbers in line, and total H2S removal was increased by 6.5%.
Plans for the future include full capacity running of the new expansion plants and replacing distributor trays in RCC absorbers. The plans will test the expected savings of 20% increase in capacity, 10% increase in H2S removal, and 500 ppm (vol) of CO2 content in RCC treated gas.
Acknowledgment
The authors thank management of Formosa Petrochemicals for approving this article as well as the support of their colleagues in the testing operation and especially the advice and assistance of Huntsman Corp.'s Arun Arunachalam and Lawrence Chu.
References
1. Bullin, J.A., Polasek, J.C., and Donnelly, S.T., "The Use of MDEA and Mixtures of Amines for Bulk CO2 Removal," Gas Processors Association Convention, Mar. 12-13, 1990, Phoenix.
2. Nagpal, S., "Designing a selective MDEA tail-gas treating unit," Hydrocarbon Processing, January, 2010, pp. 43-48.
3. DuPart, M.S., Roony, P.C., and Bacon, T.R., "Comparing laboratory and plant data for MDEA/DEA blends," Hydrocarbon Processing, April 1999, pp. 81-86.
4. Abedini, R., Koolivand Salooki, M., Esfandiari, M., and Nezhadmoghadam, A., "Seperation of H2S and CO2 from Natural Gas: Amine Solution Approach," Petroleum & Coal, Vol. 52, No. 1, 2010, pp. 40-45.
5. Michael, L.S., and Kathy, M.H., "Converting to DEA/MDEA mix ups sweetening capacity," OGJ, Aug. 12, 1996, p. 63.
6. Amiri, S., Mesgarian, R., and Firoozbakht, M.H., "Converting to a tertiary amine ups sweetening capacity at an Iranian gas facility," Hydrocarbon Processing, January 2008, pp. 47-50.
7. Dupart, M.S., Bacon, T.R., and Edwards, D.J., "Understanding corrosion in alkanolamine gas treating plants," Hydrocarbon Processing, April 1993, pp. 75-80.
8. Rooney, P.C., Bacon, T.R., and Dupart, M.S., "Effect of heat stable salts on MDEA solution corrosivity," Hydrocarbon Processing, March 1996, pp. 95-103.
9. Rooney, P.C., Dupart, M.S., and Bacon, T.R., "Effect of heat stable salts on MDEA solution corrosivity, Part 2," Hydrocarbon Processing, April 1997, pp. 65-71.
The authors
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com