Calculating high cetane 300-400° F. cut for diesel
This article provides a basis for deciding whether to evaluate a new process scheme or alter an existing one with a view toward moving a 300-400° F. cut from gasoline to diesel fuel.
Options
Average qualities for three options were calculated from a detailed gas chromatograph analysis of a reformer feed sample, along with octane and cetane numbers for individual hydrocarbons:
1. If a 300-400° F. cut were taken from the crude unit and hydrodesulfurized to less than the 15-ppm spec for low-sulfur diesel fuel, the average cetane number would be 41.
2. Extracting aromatics from the 300-400° F. leaves a premium diesel fuel blending component of 53 cetane number. The extracted aromatics could be a petrochemical feed or a 104 (R+M)/2 gasoline blending component. In either case, the economic advantage might be appreciable, depending upon the refinery's situation.
3. If paraffins were extracted separately, they would be only two cetane numbers better than the paraffin + naphthene mixture in Option 2. The remaining mixture of naphthenes and aromatics would have an average (R+M)/2 of 96.
New options for making these and other separations are described in US Patent applications 10/425,650 Apr. 30, 2003, and CIP 11/701,931 Feb. 2, 2007.
Table 1 shows percentages of the three hydrocarbon types and their average (R+M)/2 and cetane numbers used in the analysis
Percentages of the hydrocarbon types are the averages of those in C10+ fraction of 55 reformer naphtha samples from various refineries.1 The (R+M)/2 and cetane values for each type are weighted averages of values for individual hydrocarbons, using detailed GC data that were available for a particular reformer naphtha.
The 300-400° F. portion comprised 24.9% of the particular reformer naphtha, similar to the 22.8% average of 55 samples.
The 1,3,5 trimethyl benzene (mesitylene) concentration for this sample was reported as 1.10%, compared with 0.77% of 1,2,4 trimethylbenzene, which is usually dominant. Straight-run, cat-cracked, and reformate samples consistently show the 1,2,4 trimethyl benzene concentration to be much higher than the mesitylene concentration. It is assumed, therefore, that the 1.10% is in error and was changed to 0.11% for this analysis.
Individual hydrocarbon data in Table 2 include boiling point, percentage in the particular reformer feed sample, along with (R+M)/2 octane and cetane number. The octanes are from API Project data, which are available for a large number of lower boiling paraffins.2 Those data were used in estimating octanes, based on hydrocarbon structure.
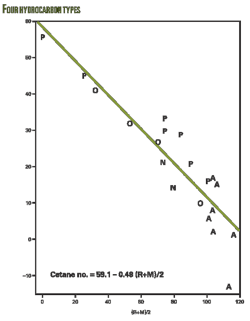
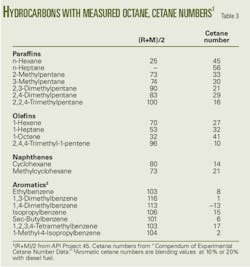
As for cetane numbers, data were available for only three aromatics and three paraffins.3 The well-known negative relationship between cetane and octane was used to estimate cetane numbers (OGJ, Dec. 3, 2007, p. 58). Because published correlations did not seem satisfactory, a correlation was developed from data on lower boiling hydrocarbons shown in Table 3 and the accompanying figure.
Here is the result of the regression analysis: Cetane number = 59.1 – 0.48 ((R+M)/2).
Comments
The author is very knowledgeable about gasoline blending but less so about process technology or economics.4 This article should be considered as a basis for deciding whether to evaluate a new process scheme or an alteration to the present process scheme.
Obviously, the exact cut points in gas chromatography do not apply to refinery operations. This is pertinent for the 4.7% normal nonane (303° F. boiling point) in the reformer feed. Elimination of the normal nonane would result in a 1.4 cetane decrease in the calculated cetane number for Option 2.
The linear calculations in this analysis do not take into account octane interactions between the hydrocarbons.5 Their effects are economically significant but small in comparison with the differences between paraffins and aromatics in this analysis.
The (R+M/2) interactions range from barely negative for some hydrocarbon combinations to significantly positive for aromatics with low-octane paraffins. Data are not available for cetane interactions, but they are likely to be opposite in sign to octane interactions, in view of the negative correlation between octane numbers and cetane numbers.
In view of the large differences in octane and cetane numbers between paraffins and aromatics, limitations in the accuracy of these estimates are probably not serious.
References
1. Unpublished data.
2. ASTM Special Technical Publication No. 225.
3. Murphy, Michael J., Taylor, Joshua E., and McCormick, Robert L., "Compendium of Experimental Cetane Number Data," NREL-540-36805, Golden, Colo.: National Renewable Energy Laboratory, September 2004.
4. www.gasolineblendingplus.com.
5. Morris, William E., "The Interaction Approach to Gasoline Blending," Paper AM-75-30, NPRA 73rd Annual Meeting, Mar. 23-25, 1975, San Antonio.
The author
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com