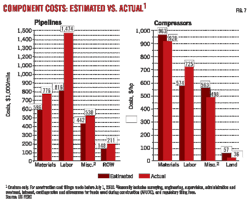
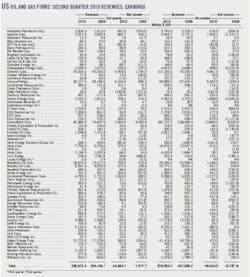
Model devised for plant hot-gas bypass systems
A method to improve design criteria commonly used to size hot-gas bypass systems has been developed through the investigation of the heat transfer that takes place at the gas-liquid interface into the tower's reflux drum.
The author used extensive plant data based on the operation of full-scale industrial depropanizer and debutanizer towers to build and validate the model, which relates the heat-transfer coefficient to the Reynolds number of liquid and gas phases.An actual plant case will be presented here, showing that the method was also useful in helping to explain major pressure instabilities caused by excessive subcooling.
Hot-gas bypass systems have been largely employed to control tower pressure in LPG fractionation in natural gas plants and light naphtha stabilization in crude distillation units. The main advantage of such a system over other pressure-control configurations is that it is able to control the tower pressure without venting or flaring any gas, since the top products—propane, propene, and butane—have high price margins. Also, the condenser is located near the ground, below the reflux drum, which is convenient for maintenance.
Even though these systems have been operating since the first half of the past century,1 2 very little is known about what happens in the reflux drum and its mathematical modeling. The work of Durand3 was the first attempt to propose a design criterion based on the energy balance around the vessel, from which resulted an expression relating the flow rates of the gas and liquid that are fed to the reflux drum. This allowed, therefore, design of the main equipment that comprise the system (control valve, condenser, and the reflux drum itself; Fig. 1).
A well-designed hot-gas bypass system should be able to control the tower pressure, in a stable way, in different operating cases, such as plant start-up, clean service of the condenser at tube-side, dirty service of the condenser at tube-side due to fouling, and reduced or increased feed rate to the tower.
Energy balance
At steady-state, all gas that is fed to the reflux drum should be completely condensed at the same rate. If the gas condensing rate exceeds the gas feed rate, the pressure will fall; if the gas feed rate exceeds the gas condensing rate, the pressure will rise. The driving force that promotes condensation in the reflux drum is the subcooling degree of the liquid phase.
The subcooling degree is the difference between the liquid saturation temperature (bubble point) and the actual temperature.
The gas phase at dewpoint releases latent heat at its saturation temperature. This energy is absorbed by the subcooled liquid phase in the form of sensible heat, raising its temperature to a value that, at maximum, will equal the saturation temperature.
Durand considered that all the subcooled liquid fed to the vessel leaves at saturation temperature,3 so that the energy balance around the vessel may be written as shown in Equations 1 and 2 (see accompanying equations box).
A conservative design will also assume the following:
• The liquid heat capacity may be determined considering the temperature T2.
• The saturation temperature at the pressure of the reflux drum T3 may be considered equal to the saturation temperature before the control valve T1.
One should notice that the saturation temperature at the reflux drum T3 is a bit smaller than the saturation temperature before the control valve T1 due to the pressure loss at the control valve, which may be calculated with Equation 3. In most cases the control valve's pressure loss does not exceed 1.0 kgf/sq cm.
Under the assumptions listed, Equation 2 will take the form of Equation 4.
It is reasonable to consider that temperature T2 may be written as the cooling water's inlet temperature Twi1 will equal temperature T2 plus the subcooling degree (Equations 5-8). It is good engineering practice not to consider an approach lower than 10° C. at the cold side of the condenser. Also, the subcooling degree should be about 5-10° C.
Equation 4 may now be written as shown in Equation 9.
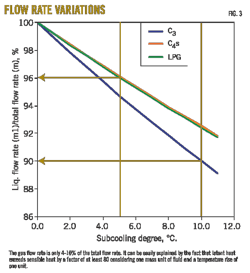
This expression enables the process design engineer to determine, as a function of the fluid properties and subcooling degree, the maximum gas flow rate fed to the reflux drum that can be completely condensed, causing all the liquid to leave it at saturation temperature (Figs. 2 and 3).
Direct contact condensation
The main problem that arises when one tries to model the heat transfer inside the reflux drum is the total lack of knowledge about the heat-transfer coefficient.
The procedure employed to determine this parameter considered that it could be calculated by the global energy balance inside the vessel, considering cocurrent flows between the gas and liquid phases and adiabatic operation of the equipment (Equation 10; Fig. 4).
With n = 1, Equation 10 becomes Equation 11, which may now be written as Equation 12. This equation permits calculation of the heat-transfer coefficient based on variables that are completely defined.
It should be noticed that the heat-transfer coefficient changes through the length of the vessel, but there are no means to calculate it as the flow rates, liquid bulk temperature, and fluid properties change. This approach brings the calculation of the heat-transfer coefficient to the entrance of the reflux drum, where all the needed variables are known.
Based on extensive plant data for an industrial depropanizer tower and according to previous studies,4-7 it was possible to develop an empirical model that showed good correlation to the heat-transfer coefficient calculated with the plant data (less than 5% difference; Fig. 5).
The empirical expression took the form of Equation 13.
It should be noticed from the adjusted parameters that the majority of the heat-flow resistance is at the liquid phase. The gas phase plays a secondary role, as would be expected, since the gas phase is almost at the same temperature.
The model was also applied to an industrial debutanizer tower in order to predict the heat-transfer coefficient, showing a difference of less than 5% between the predicted values and the calculated ones using the plant data.
Both towers belong to an 11 million cu m/day natural gas processing unit at the Duque de Caxias refinery, Rio de Janeiro, and have been operating since 2002 (Fig. 6).
Equation 13 enables solution of the energy and mass balances at each one of the n elements (Equations 14-17).
Since the boundary conditions at the entrance of the vessel are known (i=1), Equation 14 is solved for ∆mG1 and then Equations 15-16 are solved for mL1 and mG1, respectively. Finally, Equation 17 is solved for TL1.
This procedure is repeated until the last equation is solved for TLn.
A simulation was performed to the debutanizer's reflux drum showing that, at design gas flow rate, the vessel would not have enough heat-transfer area (gas-liquid interface) to condense all the feed gas considering a 5° C. subcooling and a liquid flow rate determined by Equation 9.
Notice that the thermodynamic equilibrium between the phases has not been reached, as the liquid left the reflux drum about 2° C. below the gas phase.
When the feed gas is not completely condensed, the automatic pressure controller would close the hot-gas bypass valve just enough to adjust the gas flow rate.
Process design engineers have to keep in mind that Equation 9 is very conservative when defining the feed-gas flow rate, as the vessel will not have enough heat-transfer area to reach thermodynamic equilibrium, since it is designed as hydraulic equipment and not as a heat-transfer one.
Therefore, the procedure developed may be applied to choose a feed-gas flow rate with less overdesign, leading to a more economical and rational project.
Otherwise, if a 10° C. subcooling is applied, considering the same flow rates of the last example, the feed gas is completely condensed at 2.5 m (Fig. 7).
Excessive subcooling (more than 10° C.) may create a situation in which all the feed gas is condensed before half of the vessel's length, exposing that amount of stagnant gas, which keeps the reflux drum pressurized, to an interface area that is in "excess," not required to do any condensation.
Some gas (stagnant gas) in the reflux drum cannot be condensed or else the pressure of the system would collapse. If there is an excess of interface area, which is not required to condense any of the feed gas, there is a high probability of the stagnant gas being fully condensed. It is important to remember that the condensing rate depends on both the subcooling degree and interface area.
Unfortunately, it is not possible to avoid this excess interface area from condensing the stagnant gas, which will cause serious pressure instabilities at the tower.
This same debutanizer had experienced operating problems caused by pressure fluctuations before process engineers managed to control the subcooling degree by changing the cooling water flow rate to the condenser when convenient: plant start-ups, reduced feed rates, and cold nights in severe winters (Fig. 8).
References
1. Whistler, A.M., "Locate condensers at ground level," Petroleum Refiner, Vol. 33 (1954), No. 3, pp. 173-174.
2. Boyd Jr., D.M., "Fractionation instrumentation and control," Petroleum Refiner, Vol. 27 (1948), No. 10, pp. 115-118.
3. Durand, A.A., "Sizing hot-vapors bypass valve," Chemical Engineering, Aug. 25, 1980, pp. 111-112.
4. Kim, Y., et al., "Investigation of the steam-water direct contact condensation heat transfer coefficients using interfacial transport models," Int. Comm. Heat Mass Transfer, Vol. 31 (2004), No. 3, pp. 397-408.
5. Celata, G.P., et al., "Direct contact condensation of steam on slowly moving water," Nuclear Engineering and Design, Vol. 96 (1986), No. 1, pp. 21-31.
6. Hughes, E.D., and Duffey, R.B., "Direct contact condensation and momentum transfer in turbulent separated flows," Int. J. Multiphase Flow, Vol. 17 (1991), No. 5, pp. 599-619.
7. Finkelstein, Y., and Tamir, A., "Interfacial heat transfer coefficients of various vapors in direct contact condensation," Chemical Engineering Journal, Vol. 12 (1976), No. 3, pp. 199-209.
The author
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com