Oxidative desulfurization offers route to ultralow-sulfur diesel
Heightened concerns for cleaner air are leading to more stringent regulations on sulfur content in transportation fuels. That in turn is making desulfurization in hydrocarbon processing more important.
| Here's the first formation (left photo) of the emulsion mixture (Fig. 1). The lower layer opaque emulsion (right photo) formed a white milk-like layer once the mixture temperature reached 70°C. (Fig. 2). |
The result has placed pressure not only on refiners but also on governments and legislatures. The sulfur problem is becoming more serious in general, particularly for diesel fuels, as the regulated sulfur threshold in the next few years is already aimed at virtually sulfur-free liquid.
Although conventional hydrodesulfurization is still preferred for producing ultraclean fuels, other unconventional methods–such as oxidative, radiative, extractive, membrane, adsorption, biodesulfurization, and ultrasonic–have gained interest in recent years due to increased cost of revamping existing low-pressure hydrotreating units. Most alternative technologies have not appeared to be economically viable on a commercial scale.
Oxidative desulfurization technology, however, has progressed to the point that is almost commercially ready. Oxidation chemistry represents an alternative route to diesel desulfurization that complements HDS chemistry. Integration of an oxidative desulfurization unit with a conventional hydrotreating unit can improve the economics of these regulations-driven projects relative to current HDS technology.
A few unconventional approaches to reducing sulfur content in hydrocarbon fractions include adsorption, extraction, ionic exchange, biodesulfurization, and oxidation. An effective oxidation desulfurization (ODS) catalytic system consists of sodium tungstate dehydrate (Na2WO4), aqueous hydrogen peroxide (30% H2O2), and acetic acid (CH3CO2H) has been found promising for deep removal of sulfur in diesel.
From a chemical point of view, the sulfur compounds are transferred to their corresponding sulfones, which can be preferentially extracted by polar solvents. Using ODS reduces the sulfur level in a commercial diesel to less than 39 ppm from 1,100 ppm, which meets the level in the latest stringent environmental legislation for production of ultralow-sulfur diesel (<50 ppm).
This article describes the ODS process and a proposed reaction mechanism. It also reports on testing of a new oxidation system on a 1,000-ppm commercial diesel sample supplied by Saudi Aramco's Rabigh refinery.
Reducing sulfur
Sulfur in transportation fuels remains a major source of sulfur oxide (SOx), which contributes to refinery catalyst fouling, corrosion, air pollution, and acid rain.1 2 Thus, the threshold limit for sulfur in gasoline and diesel has already been regulated globally, including Saudi Arabia, to less than 50 ppm (wt) over the next few years.1-4
New environmental legislation puts pressure on both crude oil producers and refineries to meet new regulations and to push production of ultralow-sulfur diesel (ULSD). One school of thought proposes revamping current hydrotreating facilities in refineries, while another explores new technologies that might involve either minor additions or major changes to existing refineries.
Removal of sulfur from organic sulfur compounds in liquid fuels has long been achieved by hydrodesulfurization (HDS) with a Co-Mo/Al2O3 or a Ni-Mo/Al2O3 catalyst in 320-360° C. and in 30-60 bar (435-875 psi) of H2 partial pressure.4-6 In this process and in the presence of excess hydrogen, the sulfur atom is hydrotreated to form mainly H2S as shown in the reaction of ethanethiol: C2H5SH + H2 → C2H6 + H2S.
H2S from the HDS is toxic and needs further treatment to form elemental sulfur, a nontoxic useful yellow powder. The Claus process transforms gaseous sulfur into elemental sulfur (S8). Solvent extraction utilizing a solution of diethanolamine (DEA) dissolved in water separates the H2S from the process stream.
In this process, H2S is trapped or dissolved in the DEA by bubbling a hydrocarbon gas stream containing H2S through a DEA solution. In general, conversion of the concentrated H2S into sulfur occurs in two stages:
1. Combustion of part of the H2S stream in a furnace, producing SO2, H2O, and elemental sulfur (S): 2H2S + 2O2 → SO2 + S + 2H2O.
2. Reaction of the remainder of the H2S with the combustion products in the presence of a catalyst. The H2S reacts with the SO2 to form sulfur: 2H2S + SO2 → 3S + 2H2O.
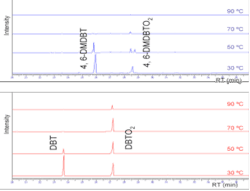
These gas chromatographs (FID) show how, under ODS treatment, both DBT and 4,6-DMDBT are oxidized to the corresponding sulfones–DBTO2 and 4,6-DMDBTO2, respectively (Fig. 3).
As the reaction products cool, the sulfur drops out of the reaction vessel in a molten state, which can be stored.
Sulfur-containing compounds that are typically present in hydrocarbon fuels include aliphatic molecules such as sulfides, disulfides, and mercaptans as well as aromatic molecules such as thiophene, benzothiophene, dibenzothiophene, and alkyl derivatives such as 4,6-dimethyl-dibenzothiophene. Those later molecules have a higher boiling point than the aliphatic ones and are consequently more abundant in higher boiling fractions.
Conventional HDS technology can desulfurize aliphatic and acyclic sulfur-containing organic compounds on an industrial scale, as in most refineries in the world. Aromatic dibenzothiophene (DBT) and especially 4,6-alkyl-substituted DBTs, however, are difficult to convert to H2S due to the sterically hindered nature of these compounds on the catalyst surface.5-7
For this reason, removal of the DBTs by HDS to give the desired low levels of sulfur in diesel requires high temperature and H2 pressure conditions and hence a larger reactor as well as an active catalyst. From environmental and economic viewpoints, it is desirable to develop a more energy-efficient desulfurization process for production of virtually sulfur-free fuel.
Deep desulfurization processes include selective adsorption,6 extraction with ionic liquids,7 oxidative desulfurization (ODS),8-11 biodesulfurization,12 and other processes.13 Due to a short reaction time at ambient conditions, high efficiency, and selectivity, ODS combined with extraction is among the more promising processes.
In this process, such sulfur-containing species as sulfides, benzothiophene, dibenzothiophene, and alkyl-related derivatives are transformed into the corresponding sulfoxides or sulfones species, which are then removed in a second step.
Various studies on the ODS process have employed different oxidizing agents, such as NO2,14 tert-butyl-hydroxide,11 and H2O2.8-11 Hydrogen peroxide is commonly used as an oxidizing reagent due to its relatively low price, environmental compatibility, and commercial availability.
H2O2 is effective in the presence a transition-metal based catalyst and in acid media.8-11 Examples of transition-metal-based systems are tungstophosphoric acid,8 Na2WO4 + [(n-C4H9)4N]Cl,15 K12WZnMn2(ZnW9O34)2 + [CH3(n-C8H17)3N]Cl,16 2-NO2C6H4SeO2H,17 hemoglobin,18 and other transition metal-based oxides.1 2 13
Here, we describe a simple and effective catalytic system for oxidation of dibenzothiophenes. The catalytic system was evaluated for the removal of sulfur-containing compounds in diesel.19-25 There is still room for improvement, however, for the catalyst and the process overall to have even better results of less than 10 ppm sulfur.
Oxidation of compounds
The catalyst system for the oxidation reaction consists of sodium tungstate (Na2WO4, 0.2 g), acetic acid (CH3CO2H, 5 ml), and hydrogen peroxide (30% H2O2/H2O, 1 ml) as oxidizing agent. In a round-bottom beaker, the oxidation reaction was carried out with separate model solutions of DBT and 4,6-DMDBT in octane (500 ppm S) at temperatures of 30°, 50°, 70°, and 90° C.
In each run, the Na2WO4 catalyst dissolved gradually in the mixture, forming first an emulsion and then an opaque lower layer with time, as shown in Fig. 1.
The lower layer opaque emulsion evolved gradually to form a white milk-like layer once the mixture temperature reached 70° C. At this temperature, a biphasic system appeared (Fig. 2).
In this biphasic system, the upper layer is clear, which has been proven to be the hydrocarbon layer (octane). The lower layer is aqueous and milk-like and contains mainly water, acetic acid, and some of the sulfones. Trace amounts of sulfone also appeared in the upper layer and most probably at the interface of both layers.
Throughout the reaction, stirring was continuous and the progress of the reaction was monitored periodically by withdrawing 0.1 ml aliquots of the upper hydrocarbon layer of the reaction mixture for gas chromatograph (flame ionization detection) and other sulfur analysis. Similar quantities were also withdrawn from the lower layer.
Every sample was immersed immediately in liquid nitrogen to suspend the oxidation reaction. Fig. 3 shows the evolution of the chromatogram of DBT and 4,6-DMDBT with the reaction temperature.
It is observed that both of DBT and 4,6-DMDBT are oxidized to the corresponding sulfones: DBTO2 and 4, 6-DMDBTO2, respectively. It can also be observed that the conversion of the sulfur compounds into sulfones increases with temperature.
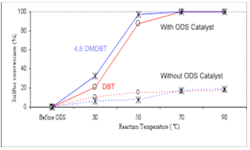
Fig. 4 shows the conversion of sulfur compounds as a function of temperature of the two model solutions, after their ODS treatment, to prove the need or otherwise for sodium tungstae catalytic system. This conversion at different reaction temperatures was calculated with the normalized peak areas as obtained from the GC-FID chromatograms.
The conversion of both DBT and 4,6-DMDBT reached its maximum of full conversion at 70° C. in less than 1 hr of reaction time. In the absence of Na2WO4, with similar amounts of H2O2/CH3CO2H, poor conversion was observed.
Shiraishi5 and Otsuki26 have calculated the electron densities of sulfur atoms for DBT and 4,6-DMDBT at 5.758 and 5.760, respectively. Such trends have been attributed to reduced availability of the lone pair of electrons and steric strain in the reaction products.
It should be worth mentioning that this new ODS catalytic system efficiently reached full conversion of the sulfur compounds to sulfones without addition of a phase transfer agent (PTA). Sato has previously reported the use of Na2WO4 with phosphoric acid and a quaternary ammonium salt promoter for the oxidation of diphenyl sulfide to give the corresponding sulfone.27 In the absence of the quaternary ammonium salt PTA, however, no oxidation was observed in the system.
Oxidation of diesel
The new oxidation system was then tested on a commercial diesel sample supplied by Rabigh refinery. The hydrotreated diesel of boiling ranges 250-350° C. has 1,100 ppm sulfur content. A selective sulfur detector, a pulse flame photometric detector (PFPD), monitored changes in the concentration of the different sulfur compounds existing in the diesel.
A series of extensive tests at various concentration levels of standard sulfur compounds established the sensitivity, linearity, and accuracy of the technique as applied to the range of sulfur compounds.
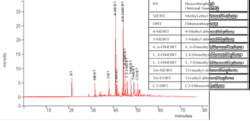
The chromatogram in Fig. 5 shows the analysis of the hydrotreated diesel sample using GC-PFPD. In this chromatogram, only the most abundant sulfur compounds according to their concentrations appear. A total of 79 different sulfur compounds was identified in this diesel, with dibenzothiophene and its alkyl derivatives being the major compounds.
In Fig. 5, the higher intensity peaks of compounds such as 4,6-DMDBT, 2-MDBT, and DBT were assigned by comparison with the fingerprint of standard samples analyzed under similar analytical conditions. The rest of the peaks were compared with several publications in which similar conditions and column specifications have been used.
The chromatogram shows that the presence of dibenzothiophene and its alkyls are dominant, since conventional HDS techniques are unable to remove these refractory sulfur compounds. In these experiments, the benzothiophene (BT) was added as an internal standard before injection in the GC-PFPD. The known concentration was used to calculate the concentration of other sulfur compounds.
The sulfur-containing compounds in the diesel sample were oxidized to their corresponding sulfones, and these were further extracted with methanol, as shown in Fig. 6. The sulfur concentration was successfully reduced by ODS and then by extraction to more than 92% and 97%, respectively.
In a reaction time of less than 1 hr, the total sulfur content in the treated diesel sample shrank to less than 39 ppm from 1,100 ppm, representing a total sulfur removal efficiency of 97%. The catalyst was mostly recovered in the aqueous lower layer and reused effectively for six consecutive new batches of ODS processes with topping up the hydrogen peroxide intake each time.
ODS reaction, mechanism
Generally, in the ODS reactions, the divalent sulfur atom of the organic sulfur compounds undergoes electrophilic addition of oxygen atoms from the hydrogen peroxide to form the sulfone, i.e., a hexavalent sulfur.
The chemical and physical properties of sulfones are much different from those of fuel oil hydrocarbons. Therefore, they can be easily removed by conventional separation such as distillation, solvent extraction, adsorption, and decomposition,10-13 as has been shown. Scheme 1 (Fig. 7) shows the overall ODS reaction and a schematic diagram of the process.
The tungstate-based catalysts have been shown effective for the oxidation of thioethers into sulfones using H2O2 as the oxidant in a two liquid-liquid phase system together with a phase-transfer catalyst (PTC).16-18
Several mechanisms of ODS reactions have been previously proposed.12 13 18 The homogeneous biphasic ODS system described here is simple and uses no PTA. Scheme 2 (Fig. 8) presents a detailed reaction mechanism.
Once the catalyst mixes with the hydrogen peroxide and the diesel fuel in acetic acid, the biphasic catalyst system starts to form at room temperature. We suggest that WO42– anion reacts in two steps with two molecules of H2O2 in sequential substitution reactions and in each step a W = O group is replaced by a W(O2) group and H2O is displaced. The resulting peroxotungstate [(–O)2 W (O2)2] anion then reacts by sequential oxygen atom transfer to the sulfur of R2S to form R2SO (sulfoxide) then the R2SO2 (sulfone), which can be extracted with the aqueous phase.
The peroxotungstate can be regenerated on the interface between the two layers or in the aqueous phase in presence of adequate supply of H2O2. Sulfones are known to be slightly more polar than sulfur compounds; so they will form a white precipitate. The whole process will result in a biphasic solution by which the upper layer becomes almost sulfur-free diesel.
The tungstate anion has the normal tetrahedral structure; Fig. 9 shows the Raman spectroscopy of the catalyst before and after the ODS reaction.
The Raman stretching above 900 cm–1 is usually attributed to the W = O stretching, while the W-O bend vibration is around 320 cm–1.20-22 The standard sodium tungstate dehydrates (STDHs) have bands at 928, 890, 836, and 331 cm–1 in its ordinary tetrahedral structure. After the ODS reaction, however, the new bands are seen at 970, 950, 895, and 312 cm–1, which suggests the presence of a different form or more than one peroxotungstate system.
Because the electrophilicity of the peroxotungstate intermediate is much higher than that of H2O2, it will participate effectively in the oxidation of sulfur atoms. The ligation on the W center would increase the reactivity of the peroxo ligands and the metal center (W) has an unchanged oxidation number of VI throughout the whole process. The sulfur compound in the form of R2S is nucleophilic due to the presence of two lone pairs of electrons on the sulfur, which can be donated to form bonds with oxygen from the peroxotungstate.
Shown is the Raman spectroscopy of the catalyst before and after the ODS reaction (Fig. 9).
The tungstate catalyst is soluble in the acid solution and forms a biphasic catalyst system. The role of the acetic acid in this reaction may be to increase dispersion of the catalyst and to promote and possibly to protonate oxo and peroxo groups of the tungstate system.9-11 14 19 25
Results
At modest temperatures and under atmospheric pressure, the ODS catalytic system, consisting of Na2WO4 H2O2 and acetic acid, is effective for removing most of the last few hundred ppm of HDS-persistent organic sulfur-containing compounds in diesel. The ODS unit, as a posttreatment unit, could therefore complement existing HDS facilities, as shown in Scheme 3 (Fig. 10).
By achieving a sulfur content of less than 50 ppm in diesel, the current ODS process, when combined with extraction, has the potential to meet future environmental regulation.1-4
Acknowledgment
The authors thank Saudi Aramco for financial support of this project and for author Shahrani's scholarship to attend Oxford University. Thanks also to Sami Barri for the diesel analysis in Imperial College. Many thanks as well to the management and colleagues in Saudi Aramco and Oxford University for their collaboration.
References
- Yang, R.T., Hernandez-Maldonado, A.J., and Yang, F.H., Desulfurization of Transportation Fuels with Zeolites Under Ambient Conditions. Science, 2003, 301 (5629), pp. 79-81.
- Gosling, C.D., Gembicki, V.A., Gatan, R.M., Cavanna, A., and Molinari, D., The role of oxidative desulfurization in an effective ULSD strategy. UOP LLC, Chemindix Bahrain, 2004.
- Turaga, U.T., and Choudhary, T.V., Desulfurization and novel process for removal of sulfur from hydrocarbons. 2006, Conocophillips. Application: WO. p. 31.
- Berti, V., and Iannibello, A., Hydrodesulfurization of Petroleum Residues: Principles and Applications. Milan: Staz. Speriment. Combust, 1975.
- Shiraishi, Y., et al., "Desulfurization of light oil by reaction of sulfur compounds with chloramine T resulting in sulfimides formation." Cambridge, UK: Chemical Communications, 2001, No. 14, pp. 1256-1257.
- McKinley, S.G., and Angelici, R.J., "Deep desulfurization by selective adsorption of dibenzothiophenes on Ag+/SBA-15 and Ag+/SiO2." Cambridge, UK: Chemical Communications, 2003 No. 20, pp. 2620-2621.
- Bosmann, A., et al., "Deep desulfurization of diesel fuel by extraction with ionic liquids." Cambridge, UK: Chemical Communications, 2001, No. 23, pp. 2494-95.
- Yazu, K., Makino, M., and Ukegawa, K., "Oxidative desulfurization of diesel oil with hydrogen peroxide in the presence of acid catalyst in diesel oil/acetic acid biphasic system." Chemistry Letters, Vol. 33 (2004), No. 10, pp. 1306-1307.
- Campos-Martin, J.M., Capel-Sanchez, M.C., and Fierro, J.L.G., "Highly efficient deep desulfurization of fuels by chemical oxidation." Green Chemistry, Vol. 6 (2004), No. 11, pp. 557-562.
- Garcia-Gutierrez, J.L., et al., "Ultra-deep oxidative desulfurization of diesel fuel with H2O2 catalyzed under mild conditions by polymolybdates supported on Al2O3." Applied Catalysis, A: General, 2006, Vol. 305, No. 1, pp. 15-20.
- Wang, D., et al., "Oxidative desulfurization of fuel oil. Part I. Oxidation of dibenzothiophenes using tert-butyl hydroperoxide," Applied Catalysis, A: General, 2003, Vol. 253, No. 1, pp. 91-99.
- Yu, B., et al., "Deep desulfurization of diesel oil and crude oils by a newly isolated Rhodococcus erythropolis strain," Applied and Environmental Microbiology, 2006, Vol. 72, No. 1, pp. 54-58.
- Song, C., and Ma, X., "New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization," Applied Catalysis, B: Environmental, 2003, Vol. 41, Nos. 1-2, pp. 207-238.
- Tam, P.S., Kittrell, J.R., and Eldridge, J.W., "Desulfurization of fuel oil by oxidation and extraction. 1. Enhancement of extraction oil yield," Industrial & Engineering Chemistry Research, 1990, Vol. 29, No. 3, pp. 321-324.
- Stec, Z., et al., "Oxidation of sulfides with H2O2 catalyzed by Na2WO4 under phase-transfer conditions," Polish Journal of Chemistry, 1996, Vol. 70, No. 9, pp. 1121-1123.
- Neumann, R. and Juwiler, D., "Oxidations with hydrogen peroxide catalyzed by the [WZnMn(II)2(ZnW9O34)2]12-polyoxometalate." Tetrahedron, 1996, Vol. 52, No. 26, pp. 8781-8788.
- Reich, H.J., Chow, F., and Peake, S.L., "Seleninic acids as catalysts for oxidations of olefins and sulfides using hydrogen peroxide," Synthesis, 1978, Vol. 4, p. 299-301.
- Klyachko, N.L., and Klibanov, A.M., "Oxidation of dibenzothiophene catalyzed by hemoglobin and other hemoproteins in various aqueous-organic media." Applied Biochemistry and Biotechnology, 1992, Vol. 37, No. 1, pp. 53-68.
- Xiao, Tiancun, Huahong, Shi, Al-Shahrani, Farhan, and Green, Malcolm, "Hydrocarbon recovery from sulfones formed by oxidative desulfurization (ODS) process." IP244-561539, Apr. 20, 2008.
- Al-Shahrani, Farhan, Xiao, Tiancun, Martinie, Gary, and Green, Malcolm, Catalytic Process for Deep Oxidative Desulfurization of Liquid Transportation Fuels. US Pat. Appl. Publ. (2007), 35 pp. WO 2007103440.
- Martinie, Gary M., Al-Shahrani, Farhan M., and Dabbousi, Bashir O., Diesel oil desulfurization by oxidation and extraction US Pat. Appl. Publ. (2007), 9 pp. US 2007051667.
- Martinie, Gary Dean, Al-Shahrani, Farhan M.A., and Dabbousi, Bashir O., Process for treating a sulfur-containing spent caustic refinery stream using a membrane electrolyser powered by a fuel cell. US Pat. Appl. Publ. (2006), 12 pp. US 2006254930.
- Martinie, Gary Dean, and Al-Shahrani, Farhan M., "Reactive extraction of sulfur compounds from hydrocarbon streams," PCT Int. Appl., 2005, WO 2005066313.
- Al-Shahrani, Farhan, Xiao, Tiancun, Llewellyn, Simon, Barri, Sami, Jiang, Jiang, Shi, Huahong, Martinie, Gary, and Green, Malcolm, "Desulfurization of diesel via the H202 oxidation of Aromatic sulfides to sulfones using a tungstate catalyst," Journal of Applied Catalysis B: Environmental 73, 2007, pp. 311-316.
- Al-Shahrani, Farhan, Oxidative desulfurization of diesel fuels. D.Phil. Dissertation, Oxford, UK: University of Oxford Press, 2008.
- Otsuki, S., Nonaka, T., Takashima, N., Qian, W., Ishihara, A., Imai, T., and Kabe, T., Energy Fuels, Vol. 14, 2000, p. 1232.
- Noyori, R., Aoki, M., and Sato, K., "Green oxidation with aqueous hydrogen peroxide," Cambridge, UK: Chemical Communications, 2003, Vol. 16, pp. 1977-1986.
Based on an article published in the Saudi Aramco Journal of Technology, Fall 2009.
The authors
Tiancun Xiao is chief scientific advisor for Oxford Catalysis Group, which he helped found. He is also an associate researcher at the University of Oxford. Xiao earned a PhD (1993) in heterogeneous catalysis from the Chinese Academy of Science. He came to Oxford University in 1999 and joined Wolfson Catalysis Centre as a Royal Society BP Amoco Research Fellow. Since then, he has been appointed visiting professor of the Beijing University of Chemical Technology, lecturing professor of Eastern China University of Science and Technology, and guest professor of Guizhou University in China.
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com