Real CO2 streams—those from CO2-capture plants likely to contain impurities as opposed to pure CO2 streams—will likely contain at least 95 mole % CO2 but will also contain impurities generated in the individual power plant and carbon capture-related facilities.
The first part of this article (OGJ, Apr. 12, 2010, pp. 39) described in detail methods for determining steady-state pressure and temperature profiles of such CO2 streams. The conclusion, presented here, addresses the expected influence of impurities present in real CO2 streams on the hydraulic pipeline layout and presents an overview diagram enabling a first estimation of the most economic pipeline diameter, depending on intended CO2 throughput rates.
Background
Type and concentration of the impurity components contained in the CO2 stream will influence the hydraulic design of a pipeline system transporting real CO2 streams, which depend on a series of considerations like:
• Power plant fuel type and carbon-capture technology.
• Health-related safety considerations referring to the maximum allowable concentration of toxic CO2 stream components (e.g., H2S, SO2) in hypothetical leak situations.
• Pipeline material-related aspects to limit corrosion (e.g., limitation of H2O concentration) or other pipe-material related adverse effects like hydrogen embrittlement of the pipeline steel, hydrogen-induced cracking, or sulfide stress cracking (which can be mitigated by appropriate pipe material selection).
• Storage requirements (e.g., concentration limitation of oxygen and noncondensable components).
• Limitation of the amount of economically usable additional components transported (e.g., thermal usage of hydrogen or methane).
• Limitation of the amount of additional components in order to minimize friction pressure losses or losses of pipeline transportation capacity.
• Limitation of the concentration of additional components in order to minimize the amount of energy required in the pipeline system's compression and transportation stations.
Impurity sources
The process or power plant application for combustion of the primary fossil fuels—coal, oil, gas, biomass, or a mixture of these—determines the CO2 capture techniques, which for power plant applications are characterized commonly as precombustion, postcombustion, or oxy-fuel processes (Table 1).
The processes mentioned may generate components appearing at different combinations and concentrations in the CO2 streams captured, H2S and SO2 resulting from the fuel's sulfur content. Table 2 gives an overview on the concentrations of the impurities expected in dried CO2 streams.1
While the stream compositions given in Table 2 reflect the aspects of the capture processes, Table 3 shows the DYNAMIS specification2 taking safety and toxicity limits into account.
The DYNAMIS report2 also states, however, that this recommendation covers a capture process applied to coproduction of electricity and hydrogen and, further, care must be used in applying this quality recommendation to other types of capture processes.
Impurity influence
Estimating the influence of impurities on the pressure and temperature profiles of a CO2 pipeline system and on the power demand of the initial compression stations and potentially installed intermediate transportation station(s) requires estimating the influence of impurities on vapor pressure-critical pressure, density, viscosity, specific heat capacity, Joule-Thomson coefficient, and isentropic p-T-relationship.
The published data on the influence of impurities on CO2 stream properties, the applicability of existing equations of state, and the applicable mixing rules and parameters data are, however, limited.3 4
The Polytec report provides example estimates for pressure and temperature-dependent density, dynamic viscosity, and vapor pressure values.3 The REFPROP program from National Institute of Standards and Technology obtained the data used by the report, referring to the statement by NIST that the program uses the most accurate equations of state currently available. The report3 comprises a compilation of available measurement data on pressure vs. temperature and vapor-liquid equilibrium data of mixtures of CO2 with other components.
Table 4 presents the influence of impurities on density, viscosity, and vapor pressure of CO2 streams at 100 bar with different temperatures, using an impurity concentration of 2%. These data were extracted graphically from the report's diagrams and are for illustration purpose only.
SO2 is the only component increasing stream density compared to pure CO2, the estimated density for this mixture is very uncertain since no mixture parameters were available. H2S has a minimal impact on the fluid density while H2 has a large impact.
Impurities typically will reduce dynamic viscosity (Table 4).
Impurities affect vapor pressure with the exception of H2S and SO2 (Table 4). The values for CO2-SO2 mixture are very uncertain, since mixing parameters were estimated and not based on actual measurement data. The presence of impurities also implies the presence of a two-phase region.3
Table 4 shows, for example, for a temperature of 30° C. (near CO2's critical temperature ~31° C.) the vapor pressure of a CO2 mixture with 2% H2 is about 8.5 bar higher than that of pure CO2.
Literature addresses the influence of impurities on critical pressure.4 Fig. 1 presents the relationships and shows variations of critical pressure of CO2 streams with different impurities.
Fig. 1 shows the increase of the critical pressure due to impurities is expected to remain moderate (<10 bar) if type and concentration of impurities remain in the ranges estimated in Table 2.
Fluid properties
Estimating the influence of impurities on the results of steady-state pressure and temperature profile calculations assumed modifications of relevant fluid properties of ±10%. Table 5 shows the results of related calculations performed for the hypothetical CO2 transportation system.
Table 5 shows variations of the CO2 stream density due to impurities as representing a dominant factor in determining pressure losses along a pipeline system. Accurate determination of the CO2 stream density regarding the presence of impurities therefore represents the major hurdle for reliable prediction of hydraulic pressure and temperature profiles along a new pipeline system for captured CO2.
The development of a new CO2 pipeline system requires estimation of the types and concentration ranges of impurity components of the CO2 stream. Tables 1 and 2 estimates for this purpose depend on the technologies applied for power generation and carbon capture.
Table 4 and Fig. 1 can estimate the critical pressure of the transported CO2 stream, defining the minimum operating pressure by considering the sufficient safety distance to the critical pressure.
Table 4 allows estimation of appropriate correction factors for density and viscosity of the CO2 stream and after selection of an appropriate pipeline diameter, first hydraulic pressure and temperature profiles can be determined applying equations for consecutive pipeline sections from the pipeline system inlet to the system outlet presented in Part 1 of this article.
This procedure provides a straightforward methodology to develop basic hydraulic pipeline profiles for new CO2 transportation systems, respecting also the influence of impurities on the calculated pressure and temperature profiles.
Economic aspects
After defining minimum operating pressure to avoid two-phase flow, minimizing specific CO2 transportation costs, including initial investment cost and energy cost to compensate the friction losses, can estimate the optimum pipeline diameter.
Assuming a constant annual CO2 throughput over the life of the project, the specific CO2 transportation cost Csp can be estimated with initial investment cost Cinv, the annuity factor a, the annual energy cost Cen, and the annual mass myr transported (Equation 1).
Annuity factor a is calculated as a function of interest rate i and number of operating years n (Equation 2).
Initial investment cost Cinv depends on parameters including pipe OD, design pressure, pipe WT, steel and coating delivery cost, and pipelaying cost. Estimates for a new CO2 pipeline system in the 16-32 in. OD range with a design pressure of about 150 bar using typical western European costs of about €39/(inch*m) yield a price for a 24-in. OD pipeline of roughly 39*24 €/m = €936/linear pipeline m.
Annual energy costs Cen are based on a determination of diameter-dependent friction losses of the specific energy costs to operate the injection-transport stations and the annual operation time of the system.
Table 6 shows the main input data used for economic calculations, assuming the CO2 stream is transported in dense phase at a density of 770 kg/cu m.
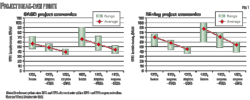
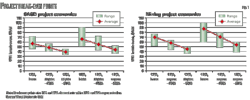
Fig. 2 shows the results of raw pipeline system optimization. For transportation of 10 million tons/year CO2, a 20-in. OD pipeline system would represent the optimum techno-economic solution. The calculated specific transportation cost equals about €1.2/ton at 100 km transportation distance. A 24-in. OD pipeline system could, however, be even more suitable if a future CO2 throughput expansion were intended (e.g., to 15 million tons/year).
The specific transportation cost shown in Fig. 2, however, reflects only the friction-loss related cost along the pipeline route. The specific cost to compress the CO2 from the capture pressure level to the dense phase has to be added separately to the specific transportation cost.
The specific shaft rated power demand for CO2 compression assuming equal stage pressure ratios as well as isentropic and mechanical efficiencies of 0.80 and 0.90, respectively, is about 366 kJ/kg (1 bar/30° C. to 80 bar) and 21 kJ/kg (80 bar/40° C. to 130 bar). Estimates for the shaft rated power demand to compress 1,200 ton/hr CO2 from 1 bar to 130 bar in the initial station measured about 122 + 7 = 129 Mw. Friction pressure losses inside the compressor station are not addressed.
Assumed specific shaft-rated energy cost of €90/Mw-hr yields a resulting specific compression energy cost of about €9.1/ton CO2 (1-80 bar) and €0.53/ton CO2 (80-130 bar). Specific annuity cost of the injection compression station is about €2/ton CO2.
The intermediate transport station's shaft-rated power demand to increase pressure to 128 bar from 88 bar is about 2.4 Mw, about 1.9% of the compression power demand of the initial station.
The curves shown in Fig. 2 provide only a rough indication of optimum diameter for a given annual CO2 throughput. Determining the optimum solution in each individual case requires more detailed calculations.
References
1. "IPCC 2005: IPCC Special Report on Carbon Dioxide Capture and Storage; Prepared by Working Group III of the Intergovernmental Panel on Climate Change," Edited by Metz, B., Davidson, O., de Coninck, H.C., Loos, M., and Meyer, L.A., pp. 442, Cambridge University Press, Cambridge and New York, NY, 2005.
2. "DYNAMIS Project No. 019672: Towards Hydrogen and Electricity Production with Carbon Capture and Storage," D 3.1.3, DYNAMIS CO2 quality recommendations, June 21, 2007.
3. Polytec Report No. POL-O-2007-138-A, "State-of-the-Art Overview of CO2 Pipeline Transport with relevance to Offshore Pipelines," Jan. 8, 2008.
4. Seevam, P.N., Race, J.M., and Downie, M.J., "Carbon dioxide pipelines for sequestration in the UK: an engineering gap analysis," Journal of Pipeline Engineering, Vol. 6, No. 3, pp. 140-141, September 2007.
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com