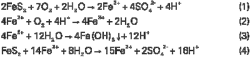Special Report: Coal seam oxidation leads to CP failure, pipeline corrosion
Coal seam oxidation under wet, high-temperature conditions caused accelerated external corrosion of a buried carbon steel natural gas flowline despite application of impressed-current cathodic protection and a liquid epoxy polymer coating.
Coal seam oxidation can create a highly acidic and corrosive environment for pipelines buried nearby. High-temperature surfaces can concentrate acidic water to an even lower pH, creating an even more corrosive environment around the pipe. Installed cathodic protection may be unable to protect pipe in an sufficiently aggressive acidic and high-temperature environment. External coating also may not resist such an environment and the accompanying increased risk of cathodic disbonding.
Background
An external coating failure and corrosion found as part of a scheduled corrosion survey forced the December 2007 closure of a 16-in. OD, 52-km natural gas flowline in southern Sumatra. The pipeline had been operating for about 11 months. A direct-current voltage gradient survey detected the coating failure and excavation found external corrosion under the coating. The corrosion site lay about 250 m away from the gas well. Multiple coating damages and pitting corrosion had caused pipe wall loss.
The pipeline consisted of API 5L X52-grade carbon steel. Operational temperature and pressure measured 90-110° C. (150° C. maximum) and 1,000-1,300 psi (6.9~9.0 MPa). The corroded pipe section lay 1.5-2 m deep in a downhill coal seam. Liquid epoxy polymer coated the pipeline's external surface. An impressed-current cathodic protection system lay at the gas-plant end of the flowline.
Site investigation and laboratory analysis showed the surrounding soil to be highly acidic. Close-interval potential survey data demonstrated cathodic-protection potential was meeting accepted standards.
The oxidation of sulfur in coal can generate a highly corrosive sulfuric acid in the soil in tropical environments. The high temperature around the pipe surface may have concentrated the acid. Literature contains limited knowledge regarding coating with CP in low-pH, high temperature conditions.
A DCVG survey showed other flowlines buried in the same area were also susceptible to external corrosion. This article highlights a research project initiated to investigate this corrosion issue and evaluate the coating stability within the CP's operational parameters.
Field investigation
The corroded section of the pipeline lay in coal seam-rich soil. Other parts of the flowline lie in the wet soil. A nearby coal seam has spontaneously combusted several times since the start of operations.
Fig. 1 shows the geographic location of the flowline.
DCVG survey and site excavation revealed coating failure and external corrosion on the pipe. Fig. 2 shows the excavation site and the soil condition.
The gas flowline was backfilled with clay soil 50-80 cm thick. Coal dominated local soil composition. Analysis showed the coal was subbituminous with total sulfur content of 0.22-1.59% and volatile matter content measuring 28-38.5%. Soils with these ranges of sulfur and volatile matter have a tendency to combust spontaneously.
A suspected coating burn was found between the 4 and 8 o'clock positions at the failure site. The coating had a dark color on its exterior and a red color on its interior (steel pipe side). Coating failure spots had either blistered or peeled off. The pipe steel surface under the failed coating spots experienced severe corrosion and metal loss.
Fig. 3 illustrates the orientation of coating failure spots on the pipe surface and where corrosion occurred. Fig. 4 shows a typical coating failure and the external corrosion's appearance.
During excavation a water sample from the ditch was collected and analyzed. Fig. 5 shows the sample collection site, and Table 1 lists the analysis results.
Fig. 6 shows results of a periodic CP survey on the flowline. The protective potential was –935 mv (Cu/CuSO4). The commonly accepted criterion for cathodic protective potential is a polarized potential more negative than –850 mv.1 2
Preliminary experiments sought to determine pH and temperature effects on CP performance and the hydrogen evolution that could contribute to the coating disbonding. A Gamry potentiostat collected dynamic polarization curves in various concentrations of sulfuric acid solution at different temperatures for the experiments.
Table 2 lists chemical composition of the X-52 pipeline steel. A Foundry-Master spark emission spectrometer obtained the results. About 40 ppm NaCl and 900 ppm Na2SO4 added to the solutions simulated the excavation site water. Table 3 lists the test solutions.
Fig. 7 shows the room-temperature polarization curves at various pH points. Fig. 8 shows the polarization curves in 0.01M H2SO4 solution at various temperatures.
Soil acidification
The subbituminous type coal at the failure site has a total sulfur content of 0.22-1.59%. Likely forms of sulfur include monosulfide (S2–), pyritic sulfur (S22–), and sulfate sulfur (SO42–). Pyrite (FeS2) is a major sulfur mineral in the coal, present in both the coal seam and sandstone strata adjacent to the coal beds at the corrosion site.
Once sulfide minerals are exposed to air and water, the oxidation process can produce sulfuric acid and ferrous sulfate. Further reaction can produce ferric sulfate, ferric hydroxide, and more sulfuric acid. Equations 1-4 (accompanying box) show the generally accepted sequence of pyrite oxidation reactions.3-5
Similarly, when the coal seam burns spontaneously, oxidized sulfur escaping as gas can combine with water in the soil to produce an acidic environment. Field analysis on the excavation ditch water showed oxidation of the sulfur in the coal generated low pH (2.54) water with SO42– being the dominant anion. The water's color may show dissolved ferric compounds to be present, but further chemical analysis would be necessary to confirm. A neutralization reaction with soil may also cause the water to contain cations dissolved from the soil.
Acidic water surrounding the hot pipe surface (90-110° C.) could be concentrated, resulting in an even lower pH. Research on acidic mine waters in California has showed negative pH and high sulfate concentration underground. Investigators believed the extreme acid water was formed primarily by pyrite oxidation and could be concentrated by evaporation with a high atmospheric temperature.6
The oxidation of the surrounding coal seam generated a highly acidic and corrosive environment for the buried pipe. The high temperature of the pipe surface could concentrate the acidic water to an even lower pH value. The coating on the external pipe surface also may not resist such an acidic environment at high temperature.
Cathodic protection
Pipelines are often coated with nonmetallic coatings, supplemented by cathodic protection to mitigate corrosion at defects and holidays in the coating. Hydroxyl ions and hydrogen will form at the surface of the cathodically protected object. These products may cause disbonding of nonmetallic coatings by mechanisms including chemical dissolution and electrochemical reduction processes at the metal-coating interface. The buildup of hydrogen pressure at this interface may also contribute to disbonding. Heat flow to the metal-coating interface can accelerate this cathodic disbanding process on components containing hot fluids.1
Coating disbondment results in more bare steel surface exposure to the environment, increasing the cathodic protection current necessary to maintain protection. The required protective current eventually may exceed the capabilities of the CP system. The steel surfaces shielded by disbonded coating debris also may not get enough current to be protected. If an acidic liquid intrudes between coating and steel surface, corrosion would be severe especially at elevated temperature.
The cathodic reaction on the steel surface with CP applied leads to reduction of the hydrogen ion in acidic environments.7 According to the Nernst equation, the hydrogen redox potential will be shifted more positive with lower pH.7 The hydrogen reduction line moves to the right at lower pH, and in sequence, the corrosion potential of steel shifts more positive, requiring more protective current to achieve the same cathodic protection level in lower pH environments.
The preliminary experiment results shown in Fig. 7 demonstrate the corrosion potential shifting more positive at a lower pH and requiring a higher protection current to maintain the CP system at the same protective potential. Higher cathodic current, however, could also generate more hydrogen, potentially exacerbate coating disbondment.
Although an industry standard mentions higher current is required for CP in an acidic environment,8 in strong acidic environments such as this, the amount of the current required and the higher risk of coating disbonding by hydrogen evolution would render CP impractical.7 CP survey data showed protection potential at the corrosion site at 950 mv, showing cathodic protection potential in compliance with industry standards. The coating and the CP, however, still failed to protect the pipe from corrosion.
Fig. 7 also shows the anodic part of the polarization curve shifting in a higher current direction at pH = 2.01, meaning a higher corrosion rate for the steel. A surface condition change of steel at this pH may cause the shift.
Industry standards also suggest pipelines operating at more than 30° C. increase their current density values.2 Although experimental results in Fig. 8 show a higher protection current as being required at elevated temperature, in this case, temperature is not as sensitive as pH to the CP operation.
Future
Testing to resolve the coating degradation and corrosion issue would involve the following research:
• Identification of effective coating and CP operation parameters to protect the pipe in low pH and high-temperature environments.
• Monitoring coating failure and external corrosion.
• Improving quality of survey data.
• Laboratory experiments to evaluate acidification and acid concentration and test the stability of the coating and cathodic disbonding at various pHs and temperatures.
• Investigating the effect of low pH and high temperature on corrosion behavior and corrosion rate of the steel in a quiescent acidic solution.
Acknowledgments
The authors thank William Thomason, Juri Kolts, Mike Joosten, Dale McIntyre, and Ronnie Summerlin for sharing their knowledge, draft review, and experimental assistance. We also thank ConocoPhillips Indonesia and the Production Assurance Technology group in ConocoPhillips Co. for their support.
References
1. Recommended Practice DNV-RP-B401, "Cathodic Protection Design," Det Norske Veritas, 2005.
2. ISO standard 15589-1, "Petroleum and natural gas industries—cathodic protection of pipeline transportation systems—Part 1: on-land pipelines," International Organization for Standardization, Switzerland, 2003.
3. Sams III, J.I., and Beer, K.M., "Effects of Coal-Mine Drainage on Stream Water Quality in the Allegheny and Monongahela River Basins—Sulfate Transport and Trends," Water-Resources Investigations Report 99-4208, US Department of the Interior and US Geological Survey, Lemoyne, Pa., 2000.
4. Pennsylvania Coal Association, "Coal mine drainage prediction and pollution prevention in Pennsylvania," Harrisburg, Pa., 1998.
5. Thomas, B.P., Fitzpatrick, R.W., Merry, R.H., and Hicks, W.S., "Acid Sulfate Soil Technical Manual (version 1.2): Coastal Acid Sulfate Soil Management Guidelines, Barker Inlet, SA," CSIRO Land and Water, Australia, 2003.
6. Nordstrom, D.K., and Alpers, C.N., "Negative pH and Extremely Acidic Mine Waters from Iron Mountain, California," Environmental Science & Technology, Vol. 34, pp. 254-258, 2000.
7. Jones, D.A., "Principles and Prevention of Corrosion," 2nd Edition, Prentice Hall, New Jersey, pp. 97, 443, 1996.
8. NACE Standard SP-0575, "Internal Cathodic Protection (CP) Systems in Oil-Treating Vessels," NACE International, Houston, 2007.
The authors
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com