Special Report: Saudi Aramco outlines FCC catalyst evaluation method
This article presents how Saudi Aramco evaluates the performance of selected fluid catalytic cracking catalysts and compares this performance with vendor projections in terms of gasoline yield, research octane number, and gasoline sulfur content.
The article describes Aramco’s FCC catalyst evaluation process for its Jeddah refinery; results for each catalyst vendors’ projections were revalidated with a microactivity test (MAT).
Based on an economic comparison and technical evaluation during the initial catalysts testing phase, we anticipated that the selected catalyst would meet the refinery’s objectives of producing gasoline with highest yield and octane number.
Actual unit operating performance confirmed the conclusions from the catalyst study.
FCC catalysts
Production of gasoline, diesel, chemicals, and other petroleum products relies heavily on catalysts. Changing FCC catalyst is one of the most common methods refiners use to improve FCC operations.
Generally, the FCC’s main objective is to crack heavy oil to gasoline and, in some cases, increase the yield of valuable light olefins, especially propylene and butylenes. Currently, FCC units supply about 30% of the world’s propylene.
Enhancing gasoline octane and LPG olefins production, especially propylene, usually involves adding a ZSM-5 based additive containing a small pore zeolite to the FCC catalyst. Due to its unique pore structure, ZSM-5 can limit access to only linear or slightly branched hydrocarbon molecules within the gasoline boiling range. ZSM-5 based additive cracks C6+ gasoline olefins to smaller olefins such as propylene and butylenes.1
The Jeddah FCC unit’s objective, however, is to maximize gasoline yield to satisfy local market requirements.
In 2004, Saudi Aramco evaluated four different technical proposals for the FCC unit in one of its refineries in order to increase gasoline yield. Some of the selection criteria for the catalyst were:
- Octane number.
- Gasoline sulfur content.
- Gasoline yield.
This article describes the catalyst evaluation process and discusses the obtained results for each catalyst.2 As part of Saudi Aramco’s evaluation procedure of new technologies, a postoperation evaluation was conducted and reported. This article also summarizes the actual operation results.3
The catalyst evaluation activity is part of an overall plan to optimize refinery operations and increase profitability. Refineries in Saudi Aramco are being audited for energy utilization. A good example is the energy optimization effort in Jeddah refinery to maximize heat recovery and minimize fuel consumption.4
FCC chemical reactions
Cracking is the process whereby complex organic molecules and heavy hydrocarbons are broken into light hydrocarbons by the breaking of carbon-carbon bonds. The rate of cracking and yield of end products strongly depend on temperature and type pf catalyst used for this cracking.
Cracking is achieved with an active zeolite-based catalyst in a short-contact time vertical or upward-sloped riser. Preheated feed of heavy hydrocarbons is sprayed into the base of the riser where it contacts the hot fluidized catalyst. Hot catalyst vaporizes the feed and catalyzes cracking reactions.
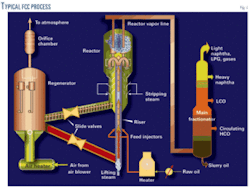
Fig. 1 demonstrates the FCC process. The FCC cracking reaction is endothermic and involves thousands of elementary reactions. Table 1 lists the most important reactions that occur in an FCC unit.5
During the cracking process, coke deposits on cracking catalyst surfaces; this causes a significant reduction of catalyst activity and selectivity. Spent catalyst is continuously removed from the cracking reactor, regenerated, and reinjected into the cracker.
The regeneration process involves catalyst contact with steam in a stripper chamber where hydrocarbons remaining in the catalyst pores are removed. Spent catalyst then flows into a fluidized-bed regenerator where air is used to burn off the coke to restore catalyst activity and also provide the necessary heat for the next reaction cycle.
The ultrastable Y-zeolite (USY) is widely used in modern FCC catalysts. It provides the main catalyst functions: product selectivity, higher stability, and much of the catalytic activity. Catalyst normally contains 20% USY and 77% amorphous alumino-silicate matrix.
Additives to the FCC process make up no more than 5 wt % of the solids but significantly improve process flexibility and product distribution.6
Yuqing investigated different USY catalysts with varying ratios of Si:Al prepared by different dealumination methods.7 After modification via lanthanum incorporation and aging at 800° C. in 100% steam for 4 hr, Yuqing characterized the pore structure and acidic property of dealuminated Y zeolites using nitrogen adsorption and pyridine-infrared methods.
Then Yuqing used a MAT to evaluate catalytic cracking performance in an FCC unit. Results showed higher cracking activity and lower coke yield in FCC unit.
Table 2 shows typical feedstock properties for the Jeddah refinery FCC unit. The main feedstock is vacuum gas oil (VGO) from the refinery vacuum distillation unit. In addition, the refinery can receive feed with slightly different properties from a nearby joint-venture refinery.
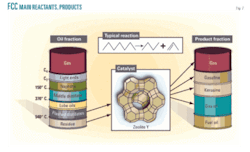
Typical feedstock properties in Table 2 represent both feeds: the refinery and exported feed. Fig. 2 shows a general illustration for the main reactants and the catalytic products for an FCC unit.
Catalyst evaluation
Changing FCC catalyst is one of the most frequently used methods chosen to improve FCC operation. In an attempt to increase the gasoline yield and octane number, we identified potential catalyst suppliers and had them submit proposals.
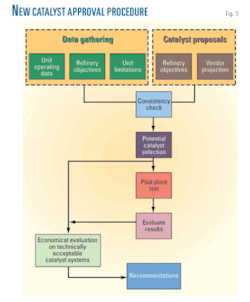
The evaluation process involves many steps to select the best FCC catalyst that meets the selection criteria contained in Saudi Aramco catalyst protocol (SAES-A-207). The flowchart in Fig. 3 describes this process in general.
In the first step, typical operating data for the FCCU (Table 3) and typical feedstock data (Table 2) were sent to several approved FCC catalyst vendors. We also requested that the vendors provide catalyst samples for our evaluation.
Three vendors sent proposals with samples of their catalysts and secrecy agreements. One of the vendors sent two samples. We evaluated four catalyst samples in total.
Table 4 shows the vendor projections for the four samples.
To validate vendors’ projections, we sent the four catalyst samples to King Fahd University of Petroleum and Minerals-Research Institute (KFUPM-RI) with a request to conduct laboratory pilot plant tests using a MAT.
Table 5 shows pilot plant test results.
Evaluation included catalyst physical properties and stability of catalyst performance within the given operating conditions. It also included the measured selectivity by MAT and octane number of the gasoline produced.
The evaluation concluded that:
- Catalyst A claimed to increase significantly LPG production. The vendor-claimed increase in gasoline was not supported by the MAT result. In addition, this catalyst was the second most expensive among the four catalysts considered.
- Catalyst B claimed a large increase in gasoline yield. The MAT results, however, did not support this claim. In addition, this catalyst was the most expensive.
- Catalyst C1 claimed a large increase in gasoline and octane number. MAT results confirmed this claimed increase.
- Catalyst C2 claimed a large increase in LPG production, which was confirmed by MAT result. This catalyst, however, did not provide a high gasoline yield.
Based on the MAT conclusions, we recommended catalyst C1 due to its high octane number and gasoline yield. This catalyst also produces gasoline with a lower sulfur content.
Actual catalyst performance
Catalyst performance was reported in first-quarter 2005 to clear the recommendation of the new catalyst. The second performance report is based on recent data, and was conducted to confirm the earlier report.
2005 initial performance test
We placed the selected catalyst into actual operation and the initial performance evaluation is based on data collected from October 2005 to March 2006 operating with the new catalyst.
During the performance evaluation, the regenerator blower was out of service for major maintenance. This caused the regenerator vessel to operate at a lower dense-bed temperature. The Jeddah refinery, therefore, started using torch oil continuously to minimize the dense-bed temperature’s effect on catalyst regeneration.
We evaluated the new catalyst’s performance based on actual operations against data obtained during the catalyst evaluation stage and vendor projections. Unavailability of the regenerator blower, however, caused two major differences in the conditions used for vendor projections and conditions used during the catalyst evaluation stage.
The two differences are the change in feedstock due to the use of torch oil and the lower operating temperature due to unavailability of additional coke burning provided by the blower.
Due to difficulties in obtaining base-case performance with the air blower down, we corrected the reported yield for the effects of low-temperature operations.
We evaluated new catalyst performance for three criteria:
Gasoline yieldCorrected = Gasoline yieldRecorded + LCO221+° C. – LCO221–° C.
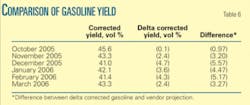
Table 6 shows a comparison between the vendor-projected yield and actual operations corrected for the low-temperature (515° C.) operating condition.
The corrected yield was lower than projected due the use of torch oil. Operating conditions during the performance evaluation were inadequate to confirm the pilot test results for projected yield. The obtained results for the new catalyst compared with historical yields for different catalysts used in the past given the same conditions, however, supported the pilot test conclusions.
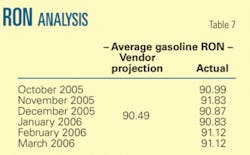
The average reported RON for all months are higher than the vendor projected.
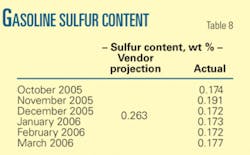
The average reported sulfur content for all months are much lower than vendor projections.
Catalyst performance confirmation
The catalyst performance methodology we followed was based on correcting gasoline yield for different operating conditions than the ones initially used to project FCC yield. This methodology was necessary to complete a mandatory procedure set by Aramco to approve a conversion to a new catalyst.
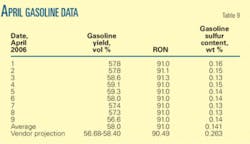
We attempted to confirm our previous conclusions based on recent performance operating the FCCU under normal operating conditions. Table 9 shows daily average yield, RON, and gasoline sulfur content for the first 9 days in April 2007.
These data show that the new catalyst performance meets and even exceeds vendor projections. Gasoline yields during this period were 56.6 wt % to 59.3 wt %.
The variation in gasoline yields is due to different blends of local and imported VGO feeds. Imported VGO slightly reduces the gasoline yields. Yields using the new catalyst are higher than the original catalyst’s yield, which was less than 57.8 wt %.
The new catalyst provides a higher RON (91) compared with the vendor projection (90.4). The new catalyst improved gasoline quality compared with the original catalyst RON of 90.
Actual measured sulfur content of 0.14 vol % is much lower than the vendor projection of 0.263 vol % and original catalyst performance of 0.3 vol %.
Economic benefits
The four catalysts showed different projections and, based on Saudi Aramco’s selection criteria, we selected the catalyst that satisfied most of the refinery criteria. The selected catalyst, therefore, might not be the best choice for another refinery with different target product.
This article shows that many factors influence gasoline yield, including riser outlet temperature, feedstock quality, and catalyst formulation.
Based on an economic comparison and technical evaluation during the initial catalyst testing phase, we anticipated that the selected catalyst would meet the refinery’s objectives of the highest yield and octane number for the gasoline stream. Actual performance confirmed the conclusion that we reached during the study phase.
Acknowledgments
The authors acknowledge the support and cooperation provided by the downstream process engineering division, process and control systems department; FCC engineers from the operation engineering unit, Jeddah refinery department; and KFUPM-Research Institute.
References
- Fu, J., and Xu, M., “Using ZSM-5 Additive with DMS based FCC Catalyst for Increased Propylene Production,” presented at the 7th International Symposium on Advances in FCC, American Chemical Society 232nd Meeting, San Francisco, Sept. 10-14, 2006.
- Al-Alloush, S., “Catalyst Management Protocols in Saudi Aramco: Case Study: Fluid Catalytic Cracking Catalyst Selection,” presented at the 6th Russia & CIS Refining Technology Conference, Moscow, Sept. 27-28, 2006.
- “FCC Performance Report,” Saudi Aramco process and control systems department.
- Al-Khaldi, Fahad, “Energy Optimization For JR FCC unit,” presented at the 3rd International Energy, Exergy, and Environment Symposium, Évora, Portugal, July 1-5, 2007.
- Sadeghbeigi, R., Fluid Catalyst Cracking Handbook, 2nd Ed., Gulf Professional Publishing: Houston, 2000, p. 126.
- Al-Alloush, S.S., Yeh, G.J., and Aitani, A.M., “Overview of Catalyst Management and Selection Protocols at Saudi Aramco’s Refineries,” Saudi Aramco Journal of Technology, Fall 2006.
- Zhang, J., et al., “Dealuminized Y zeolite cracking catalyst for petroleum residue,” Ranliao Huaxue Xuebao, Vol. 34 (2006), No. 3, pp. 319-23.
Suppliers raise refining, petchem catalyst prices
David N. Nakamura
Refining/Petrochemical Editor
During the past 3 months, catalyst manufacturers have announced significant price increases for refining and petrochemical catalysts in response to inflation and higher raw material and energy costs.
On July 23, Grace Davison announced that it was implementing price increases and surcharges of up to 20% across product lines due to “unprecedented raw material and energy inflationary pressures.”
The increase was in addition to earlier announcements for specific product price increases. “Previous measures are insufficient against the current trend of rapidly escalating costs,” according to Greg Poling, President of Grace Davison.
Haldor Topsoe, on July 25, announced that prices for its selective catalyst reduction DeNOx catalysts would rise 10-15% due to significant increases in raw material and energy costs.
On Aug. 7, Albemarle Corp. announced price increases of 20% for its FCC catalysts for both heavy feed (resid) and vacuum gas oil applications.
On Sept. 9, UOP LLC announced that it was increasing prices for all of its catalysts used in refining and petrochemical production. Increases of up to15% affect its Platforming, Penex, Unicracking, and Merox refining catalysts as well as its Parex, Isomar, Tatoray, Pacol, Oleflex, Q-Max, and EBOne petrochemical catalysts.
UOP is raising prices due to “the continued high cost of energy, packaging, and rising raw material prices.” Price increases vary by the type of product.
The authors
Saeed S. Al-Alloush ([email protected]) is a senior process engineer in the downstream process engineering division, process & control systems department for Saudi Aramco, Jeddah. He has 15 years’ experience with Saudi Aramco in refining, mainly in FCC. Alloush holds an MS in engineering from the University of Tulsa. He is a member of AIChE.
Fahad A. Al-Khaldi is a planning specialist in Saudi Aramco’s facilities planning department, Jeddah. He has contributed to the design and optimization of the utility systems and cogeneration design development for many Saudi Aramco projects. He holds a BSc (1988) in chemical engineering, an MBA (1992), and an MSc (1996) in water resource and environmental engineering, all from King Fahd University of Petroleum & Minerals, Saudi Arabia. Khaldi is currently a part-time PhD student at KFUPM.