Adjustments to a pipeline’s cathodic-protection program can help address possible hydrogen diffusion brought about by nonconformance with welding procedure specifications (WPS) during construction.
Low-hydrogen welding processes like gas metal arc welding (GMAW), flux-cored arc welding (FCAW), or where necessary selection of low-hydrogen shielded metal arc welding (SMAW) consumables are required for joining high-strength steel pipeline material, particularly if the structure is also protected by cathodic protection.
Deep-digging arc characteristics of cellulose-coated electrodes allow better weld metal penetration and arc control in varying welding positions as compared with other available pipeline welding processes. The use of cellulose-coated electrodes, however, increases hydrogen content in the weld metal, conflicting with requirements for low-hydrogen weld metal during welding of high-strength steels. Increased demand for higher-strength steels has heightened the importance for controlling diffusible hydrogen levels in weld metals.
This article examines resolution of this problem in one pipeline project.
Background
Construction of a 1,000-mile large diameter gas pipeline involved major river crossings, steep rises in elevation, and passage through environmentally sensitive areas.
Measures taken to reduce construction’s effect on the environment included applying 3 LPE coating to the pipe and providing both completed sections and the finished pipeline with cathodic protection.
The pipeline used API 5L X-70 PSL2 line pipe, with an average yield strength greater than 79,000 psi.
The pipeline’s operator asked its contractors to use a low-hydrogen welding process. These contractors qualified the welding procedures with a GMAW semiautomatic welding processes for construction, and a combination of FCAW and low-hydrogen SMAW for repairs, tie-in, and welds that could not be completed semiautomatically.
Except for the FCAW process, all weld processes had a certified diffusible hydrogen content of 4 ml/100 g of weld metal. The FCAW process accepted a maximum diffusible content of 8 ml/100 g of weld metal, in line with American Welding Society AWS A5.29 and AWS A5.20 requirements.
Construction lasting several months required cathodic protection of any pipe lying in soil for long periods between installation and start of operations. Cathodic-protection installation occurred in two phases:
- A temporary CP system with sacrificial anodes to provide external corrosion protection during construction.
- A permanent system with an impressed current to protect the pipeline for its design life (acceptance criteria for CP polarization of –0.95 v to –1.5 v).
Spur construction took place in parallel with construction of the main pipeline. Local contractors under a different construction and quality management team built these spur lines. The spur line used the same grade pipe as the mainline but measured 36-in. OD and 0.75-in WT.
An internal audit discovered the local contractor for this spur line had used a previously approved WPS from an unrelated job not suitable for this section of the pipeline. A roughly 390-ft section of pipeline consisting of 32 welds used this non-conforming WPS. The WPS allowed the contractor to use an SMAW process with E-6010 cellulose-coated electrodes for root pass and hot pass and E-8010 G electrodes for the rest of the weld.
On detecting these deviations, field inspectors issued a nonconformance order and quarantined this section of pipe pending resolution by engineering.
The problem
Construction management and engineering had to decide whether to:
- Accept the weld as is.
- Reject the welds and ask the contractor to reweld the section.
- Find an engineering solution to compensate as much as possible for the extra hydrogen these welds could contain.
Construction management placed a premium on a rapid solution.
The contractor accepted that the use of the chosen WPS was wrong but disputed both that the weld would have any adverse effect and that it would contain sufficient diffusible hydrogen to warrant cutting away and rewelding the section.
Engineering began to review material test reports from pipe mills. Five different heats of pipe were discovered, with yield strengths of 77,010, 77,889, and 81,790 psi: an average of 78,896.33 psi, close to the average yield strength of the mainline pipes.
Engineering next analyzed a weld sample for the presence of diffusible hydrogen. If the amount of hydrogen found was less than 16 ml/100 g of weld metal, the welds would be retained.
The contractor agreed to participate in this test and abide by the results. Under supervision of engineering, the contractor’s welders assembled three weld sections of the project pipe with cellulose-covered welding electrode A5.5, E 8010 G, batch number SI 7684934, the same type used for the welding of the quarantined section of pipeline.
Testing
MET-HEAT Engineers Pvt. Ltd. cut four specimens from the three welds and analyzed them according to AWS A4.3 (Rev. 2006), determining the amount of diffusible hydrogen contained by standard mercury displacement method. They maintained a bath temperature of 45° C. ±1° C., allowing a 72-hr hydrogen evolution time. All three welds contained more than 16 ml diffusible hydrogen/100 g weld metal, averaging 40.89 ml/100 g (see box).
This diffusible hydrogen exists in atomic form (also called nascent hydrogen), as opposed to the molecular hydrogen associated with such weld imperfections as gas pockets and porosities. Molecular hydrogen cannot diffuse in the steel substrate and is hence relatively less harmful.
Hydrogen diffusion in metals occurs when a hydrogen molecule dissolves on a metal’s surface. The hydrogen molecule dissociates into two atoms (Sieverts’ Law), incorporated by the bulk solid via diffusion through interstitial sites in the metal crystal lattice.
A dissolved hydrogen atom creates a lattice defect capable of inelastically scattering phonons, the thermal oscillation of an atom. In a scattering event, energy in the phonon transfers to the hydrogen atom, forcing it over a potential energy barrier and propagating it through the lattice as a free wave. This released potential energy, if not absorbed by the ductility of the material, exceeds the yield strength of the material and causes it to crack.
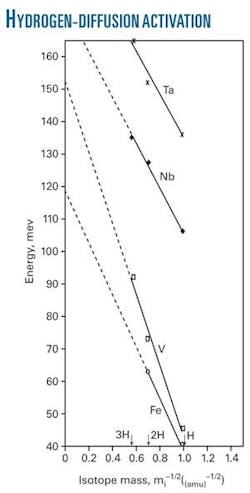
The accompanying figure plots the activation energies for hydrogen isotope diffusion in metals, Fe, V, Nb, and Ta as functions of isotope mass. The data form the straight lines predicted by the theory, proving the isotope effect in the activation energy is due to hydrogen-atom zero point vibration at an interstitial site.
MET-HEAT also considered the additional hydrogen evolved through cathodic protection, in excess of the hydrogen present in the weld metal. Atomic hydrogen evolves as the natural reaction product of both corrosion and cathodic protection. The partial cathodic reaction 2H+ + 2e → H2 shows the evolution of hydrogen gas.
Review of the specifications led to limits on adding additional hydrogen beyond what was present in the weld metal. Redesigning the polarization current for cathodic protection limited the evolution of excess hydrogen, consistent with NACE RP 0169 Sec. 6, Para. 6.2.2.3.4.1
Initial project design defined cathodic-protection requirements in the range of –0.95 v to –1.5 v. for the entire pipeline. Engineers suggested the close interval potential (CIP) survey data for the quarantined section of pipeline be reviewed for any possible changes in an effort to reduce the hydrogen evaluation for this section of the pipeline.
CIP survey data supported making special conditions for this section of the pipeline, adjusting the impressed polarization current to a range of –0.85 v to –1.1 v.2
The pipeline operator asked the CP contractor to adjust the transformer control to achieve the suggested polarization range. Follow-up CIP surveys established achievement of required protection to the pipe section. The operator plans additional monitoring of this section of pipeline by its integrity-management group and periodic review by engineering as a precaution.
References
- NACE RP0169, “Standard Recommended Practice: Control of External Corrosion on Underground or Submerged Metallic Piping Systems,” Sec. 6, Para. 6.2.2.3.4, National Association of Corrosion Engineers, 2002.
- NACE RP0169, “Standard Recommended Practice: Control of External Corrosion on Underground or Submerged Metallic Piping Systems,” Sec. 6, Para. 6.2.21.2, 6.2.2.1.3, and 6.2.2.3.4, National Association of Corrosion Engineers, 2002.
The author
Ramesh Singh ([email protected]) is a senior principal engineer, specializing in materials and corrosion, at Gulf Interstate Engineering Co. He graduated from California Coast University with an MS (2003) in engineering management and gained his basic metallurgical education (1984) from Air Force Technical College, India. He is an Incorporated Engineer, registered with the Engineering Council in the UK, and Member of The Welding Institute, Cambridge, UK.