Fossil fuel development requires long-term sulfur strategies
Based on history, the world will require long-term sulfur management strategies because the increased development of fossil fuels likely will result in involuntary production of large amounts of surplus sulfur.
At present, the high price for sulfur has removed the stranded label from even the most remote sulfur blocks. Nonetheless, experience has shown that this situation may not last. If that surplus arises, it may persist for long time in comparison with the historical frequency of up and down cycles of the sulfur market.
Current management strategies have dealt with surplus sulfur, for the most part, satisfactorily over the short-term. These strategies, may be characterized, in part, by:
- Relatively short-term storage, during which older blocks have been preserved from the initial pour of about 25 years.
- Magnitude and duration of existing acid-gas injection schemes.
- Capacity of newly developed sulfur consuming products to use large amounts of sulfur relative to what may be produced.
If one considers that a period of sulfur surplus could extend as long as our dependence on fossil fuel consumption continues, the limitations associated with current strategies come into focus. These limitations arise from many factors, including the inherent physical and chemical properties of sulfur as well as regional variations in regulatory frameworks, prevalent climatic conditions, environmental impact tolerance, and available space at grade, below grade, or in subterranean formations.
In recent years, Alberta Sulphur Research Ltd. has been examining the chemistry associated with several concepts that deal with long-term management of surplus sulfur.
Concerning aboveground block storage, the company has examined certain protective barriers that may help preserve the physical and chemical integrity of the sulfur block. It has also investigated in laboratory and field demonstrations the feasibility and benefits of storing sulfur in shallow below-grade excavations with subsequent capping. Another study looked at certain aspects of acid-gas injection chemistry for facilitating modeling of the behavior of fluids rich in H2S and CO2.
Finally, it has investigated a new use for sulfur in which hydrogen sulfide is combusted to sulfur dioxide, the thermal energy captured, and the sulfur (and perhaps some CO2) sequestered by injection into, for example, a depleted sour-gas reservoir.
Blocked sulfur storage
Much of the elemental sulfur recovered from oil and gas operations store & eventually is as calcium sulfate following its use as a raw material for producing sulfuric acid, most of which is used in producing phosphate fertilizer.
Storing sulfur produced from a Claus plant in excess of marketable amounts usually involves formation of large solid sulfur blocks created by a staged pour of molten sulfur within aluminum forms.
Various references document best practices for preparation of proper bases (topography, load bearing capacity, footprint, and materials of construction), pouring equipment and procedures, block geometry and acid run-off management.1-3 Alberta’s Energy Resources Conservation Board documents IL 84-11, GB 92-4 and Directive 055 Regulatory specify matters concerning sulfur storage.4-6
As a whole, Western Canadian operating companies and the sulfur technology service sector, together with the regulatory framework that guides and oversees both blocking and remelt operations, have demonstrated that for intermediate periods of time, sulfur storage in above grade blocks can be engineered in an economically sound and environmentally friendly manner. The structure in Fig. 1 is a good example of such an engineered block.
In long-term storage, however, the physical properties of sulfur combined with continuous exposure to elements creates a porous structure susceptible to physical breakdown. Upon solidification, elemental sulfur undergoes a subsequent phase change within the solid from a monoclinic to an orthorhombic crystalline configuration. The latter has a higher bulk density than its monoclinic counterpart; thus, cooling to ambient conditions over several hours creates internal stresses.
Release of these stresses cause fissures to develop, from microcracks to large fractures, that enhance porosity and make the block somewhat brittle.
Climatic forces such as abrasion from wind-born dust and sand, erosion from rainfall percolation and runoff, and fracture propagation from sequential freeze-thaw cycles can deteriorate the material. Perhaps the bacterial activity that leads to acid production may also play a role in the breakdown of solid sulfur.
During 20 years or more, the environment’s action on the mineral can severely compromise the structural integrity of a block. Fig. 2 shows an example of such cumulative effects on a block.
If aboveground blocking of sulfur remains an option for storing, not for 20 years but for 200 years, one might expect the condition of the block to be somewhat unrecognizable if left exposed to such forces. One should note that the effects of sulfur on the environment, in terms of dust liberation and acid runoff, would become exaggerated as the surface area of the block increases from structural breakdown.
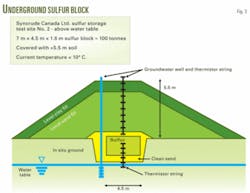
In recent years ASRL has investigated two approaches for mitigating the influences of these factors on the long-term integrity of sulfur blocks. The first involves placing the block below grade and capping it with suitable materials for controlling one or more environmental conditions. A field test examined this concept at the Syncrude Canada Ltd. oil sands mine at Mildred Lake (Fig. 3).
Burial to slightly more than 5 m will reduce the temperature of a small block to 5° C., ±3° C. (Fig. 4), and reduce the flux of water by use of high-density clays for the sloped side walls and capping layers. If the block is above the water table, water flux is even easier to control.
The control of temperature and water in such a manner may reduce bacterial activity of thiobacilli to low levels and completely eliminate freeze-thaw cycles.
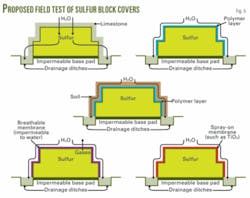
A second possible strategy for preserving the integrity of sulfur blocks is to cover the finished block surface with a protective layer of material that will act as a barrier to the elements. ASRL has examined in laboratory scale two such barriers: limestone and stucco.
Limestone rock has the benefit of reducing surface temperatures on the block and neutralizing some of the acidity that may be generated at the surface. According to Crescenzi, the resulting salt may also act as a biocide to inhibit further bacterial action.7
A pilot program, initiated at two locations, is testing various types of barriers or coatings. In 2005, Syncrude Canada Ltd. poured miniblocks at their mine site in Fort McMurray to test four barriers together with an unprotected block. An overseas company also has initiated a pilot investigation of covered-block storage. ASRL hopes to initiate a similar field test in south central Alberta within the year (Fig. 5).
The concept of a protective strategy will not, in all likelihood, relieve the owner of the block from a requirement to collect and possibly even treat runoff before release to the environment. Furthermore, the block may require periodic maintenance to preserve the integrity of the barrier over many years.
This would seem a suitable business for third-party contracting following closure of a mine.
Acid-gas injection
Since 1989, companies in Western Canada have disposed of acid gas by injecting it into subterranean formations. Target reservoirs have consisted of deep saline aquifers, depleted sour-gas reservoirs, and at least one active sour-oil reservoir.
To date the largest operation in Canada is the Talisman-Spectra operation at Kwoen in northeast British Columbia. This operation injects close to 660 tonnes/day (tpd) sulfur (as H2S) and 320 tpd of CO2.
The ERCB reported in its 2006 Annual Report on Sulphur Recovery that 38 acid-gas injection schemes were in operation in Alberta with total licensed injection capacity of 572 tpd sulfur.8 Furthermore, the same document reports that of the 14 sour-gas processing plants degrandfathered during 2001-06, half selected acid-gas injection as the H2S-CO2 management option.
While most schemes to date are small in scale, relatively few technical obstacles exist for scaling up from the 1.7 million cu m/day operation at Labarge in Wyoming to much larger operations compressing raw sour gas or amine acid gas.
The sequestration of H2S by aqueous phase trapping, mineralization, and mobile-phase migration have been studied, but some concerns remain regarding long-term containment.
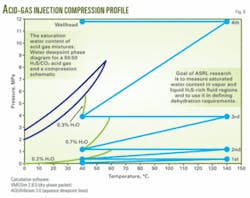
One area that required further research was the generation of additional phase-equilibrium data to predict dewpoint temperature and pressure of various acid-gas compositions for the injection. The prediction would enable the design engineer to decide on the requirement for active dehydration or, conversely, use of stainless steel for construction of the pipeline to the injection well.
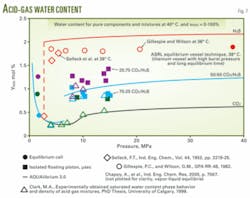
In June 2007, ASRL presented more than 150 new experimental data points for the acid gas and water system covering conditions illustrated in Fig. 6. Fig. 7 plots data generated at 40° C. together with literature data for the pure-component moisture capacity.
Alami recently analyzed these results with a semiempirical model.9
New sour-gas processing developments that are relevant to acid-gas injection schemes include acid-gas separation technologies that result in a high-pressure, acid-gas stream (actually a dense-phase fluid or a subcooled liquid) off the back end. This, of course, saves on compression horsepower requirements.
These developments include the Kvaerner Cryogenic Fractionation scheme,10 the ExxonMobil Controlled Freeze Zone (CFZ) process,11 and the Total Sprex process.12
Acid-gas combustion
One specific drawback to acid-gas injection is that hydrogen sulfide returned to the reservoir is an energy-rich molecule.
By foregoing the Claus operation, the plant loses a source of steam that is typically used to regenerate the rich-amine stream.
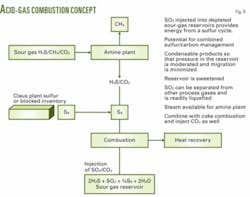
Clark has proposed a one way of recovering that energy and returning sulfur to the reservoir.13 The method is to combust the acid gas to sulfur dioxide and compress and liquefy the SO2 stream and inject it downhole. The target reservoir would be a depleted sour gas or a sour-oil formation.
Once the SO2 or the SO2-CO2 mixture reaches the reservoir, the SO2 will react almost instantaneously with the residual hydrogen sulfide in an underground Claus reaction. This reaction will produce two condensable products: sulfur and water. These products should not migrate laterally to any extent.
Depending on the dominant sequestration mechanism, the pressure in the reservoir may not increase but could decrease in time. The H2S content of the reservoir fluid also will decrease in time. Thus, this is an opportunity to take a sour-hydrocarbon resource, generate sweet gas with no sulfur emission and no carbon emission to generate steam or electricity from the combustion and sequester the SO2 and CO2 downhole.
Downhole there is little pressure build up, and the process eliminates long-term concerns about cap rock or wellbore integrity. Fig. 8 illustrates the concept. Of course, one could burn the surplus sulfur to generate heat and SO2 as well. An engineering company has already proposed a two-step combustion process.14
An intensive program in the laboratory has focused on this concept, which ASRL presented in 1999. To date, the program has examined the properties of sulfur (melting point and viscosity under high acid-gas partial pressure) and the properties of SO2-CO2 mixtures under elevated temperature and pressure (density, solubility in aqueous fluids, and saturated water content).15 16
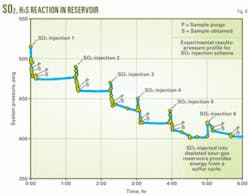
Fig. 9 shows evidence of the rate of reaction between the residual H2S and the injected SO2. This plot shows pressure vs. time for a set of sequential injections of SO2 into an autoclave containing sour gas.
The transducer trace picks up the momentary increase in pressure following each injection event, followed by the drop in pressure. The investigation also sampled the headspace and analyzed it following each injection to determine the presence of either reactant following the reaction.
The work also has observed the progress of the experiment, as given by the pressure trace, through a sapphire-windowed cell in the ASRL laboratory, and the reaction is fast. In this case, the system temperature was 125° C. and the initial pressure was about 475 psi.
Currently the concept is the subject of a joint-venture special project to conduct core flood experiments for generating viscosity data for SO2 or SO2-CO2 mixtures under pressure and to model the dynamics of the injection scheme within the wellbore and reservoir.
Topside engineering design and economic study will round out Phase 1 of the project. Phase 2 will consist of a field demonstration of the injection itself.
References
- Doyle, K.B., “Pouring Sulfur to Block Inventory, The Canadian Strategy and Experience,” IFA Production and International Trade Committee Meeting, Dublin, Ireland, Oct. 12-13, 1993.
- Hyne, J.B., “Base Pad Preparation, Pouring to Block and Related Matters,” ASRL Quarterly Bulletin, Vol. XXVIII, No. 1-2, April-September 1991, pp. 15-28,
- Irani, J.P., et al., “Pouring Sulfur to Block and Future Reclaim Lessons Learned,” Sulphur 99, Calgary, Oct. 17-20, 1999.
- Approval, Monitoring and Control of Sulphur Storage Sites, ERCB Information Letter 84-11, Oct. 29, 1984.
- Requirements for Sulphur Storage Facilities, ERCB General Bulletin 92-4, Mar. 10, 1992.
- Storage Requirements for the Upstream Petroleum Industry, ERCB Directive 055, December 2001.
- Crescenzi, F., et al., Method for Inhibiting the Biological Acidification of Water in Contact with Materials Containing Sulfur,” US Patent 20050002825.
- Sulphur Recovery and Sulphur Emissions at Alberta Sour Gas Plants, Annual Report ERCB ST101-2007, July 27, 2007.
- Alami, I., et al., “Equilibrium Water Content of High Pressure Acid Gas: A Mathematical Model,” 4th International Conference on Sour Oil & Gas Advanced Technology, Abu Dhabi, Apr. 29-30, 2008.
- Sterner, T., “Acid Gas Fractionation,” Laurance Reid Gas Conditioning Conference, Norman, Okla., Feb. 26-28, 2001.
- Valencia, J.A., and Howard, R., “The Controlled Freeze Zone Technology for Lower Cost Processing of High CO2 and H2S Gas,” 4th International Conference on Sour Oil & Gas Advanced Technology, Abu Dhabi, Apr. 29-30, 2008.
- Lallemand, F., et al., “Highly Sour Gas Processing: H2S Bulk Removal with the Sprex Process,” 2nd International Conference on Sour Oil & Gas Advanced Technology, Abu Dhabi, Nov. 27-28, 2005.
- Clark, P.D., and Davis, P., “SO2 Injection: An Energy-Efficient Strategy for Dealing with H2S in Sour Gas Production,”Sulphur 2000, San Francisco, Oct. 29-Nov. 1, 2000.
- Clark, P.D., and Stevens, D.K., “Method for Energy Recovery from Hydrogen Sulfide,” US Patent 7,282,193.
- Davis, P.M., et al., “SO2 Injection into Depleted Sour Gas Formations—Experimental Studies on Properties of Sulfur under Reservoir Conditions,” ASRL Semi-Annual Research Review, Jan. 26, 2005,
- Davis, P.M., et al., “SO2 Injection into Depleted Sour Gas Formations—Experimental Studies on Properties of SO2/CO2 Mixtures and the Rate of Reaction of SO2 with H2S Under Reservoir Conditions,” ASRL Semi-Annual Research Review, June 22, 2005.
Based on a presentation to PennWell’s Oil Sands and Heavy Oil Technologies conference, Calgary, July 16-17, 2008.
The authors
Paul Davis is general manager of Alberta Sulphur Research Ltd. at the University of Calgary. During his 24 years at ASRL, he has worked on sour gas production chemistry and has conducted extensive studies of the phase behavior and physical properties of sulfur and H2S-rich systems at elevated temperatures and pressures. Davis is a graduate of the chemical engineering program at the Southern Alberta Institute of Technology.
Rob Marriott is an experimental physical chemist who has been a project manager in upstream research at ASRL since 2004. His work involves various aspects of sour gas flow assurance, reservoir fluid properties, and sour gas-surface interactions. His current research interests include high-pressure sulfur chemistry, solution thermodynamics, and fundamental thermodynamic H2S-sulfur interactions.
Ed Fitzpatrick is a research chemist with ASRL, specializing in experimental investigations of sour gas chemistry at elevated and subambient temperatures and pressures. His work includes sulfur deposition studies and water content measurements in H2S-rich fluids. Fitzpatrick has a chemical technology diploma from the Northern Alberta Institute of Technology.
Herman Wan joined ASRL in 2003 and has specialized in properties of formed sulfur, long-term sulfur management strategies, emissions from sulfur blocks, and other uses of sulfur. Prior to joining ASRL, he has experience with analytical chemistry in the environmental and biotechnology fields, and has assisted in academic research projects pertaining to groundwater chemistry. Wan has a BSc in environmental science from the University of Calgary.
Francis Bernard has worked at ASRL for 8 years and is involved in projects related to corrosion, sulfur emissions, sulfur storage, and new uses of sulfur. He specializes in analytical chemistry, sulfur deposition, and high-pressure sour gas measurements. Prior to joining ASRL, he was developing acidizing and fracturing formulations for the oil and gas service industry. Bernard has a BSc in chemistry from the Université de Sherbrooke.
Peter Clark is professor of chemistry at the University of Calgary and technical manager of Alberta Sulphur Research Ltd. From 1995 to 2005, he directed the activities of ASRL. Clark was appointed as a Natural Sciences and Engineering Research Council of Canada Industrial Research Professor in 1986 in association with Alberta Sulphur Research Ltd. at the University of Calgary. He received PhD and BSc degrees in chemistry from the University of Hull, East Yorkshire.