Heat-stable salts reduce H2S leaking from amine absorbers
An amine heat stable salt (HSS) is the thermally unregenerable protonated form of the amine. The HSS is itself a product of the neutralization reaction between the alkaline amine and an organic or inorganic acid. Such acids enter the solution either as the result of degradation of the amine or by absorption of sulfur oxides or other acid-forming components from the raw gas.
The presence of amine HSSs can cause poor sour-gas contactor and treating plant performance. The existence of HSSs is not, however, always detrimental to amine unit performance. Under certain circumstances, HSSs can actually help achieve reductions in H2S leaking from absorbers.
This article recounts our experiences with solution switching from diethanolamine (DEA) to methyldiethanolamine (MDEA) and the effects of HSSs generation on our system performance.
System performance
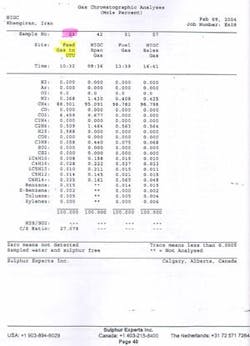
Shahid Hashemi Nejad (Khangiran) Gas Co. lies in northeast Iran, 40 km from the Turkmenistan border. The sour-gas sweetening facilities based on amine are designed to treat natural gas with about 10% acid gas from Mozdouran gas field (Fig. 1).
Each of three trains (Gas Treatment Units 1, 2, and 3) was designed to treat 346,000 normal cu m/hr (300 MMscfd) sour gas with 910 cu m/hr DEA and constructed about 30 years ago.
Two trains, GTU Nos. 4 and 5, were added 5 years ago and are identical with previous trains. All were operating with DEA as solvent. A year ago after experimental stages, all trains were switched from DEA to MDEA.
Operating on MDEA allows Khangiran to process 420,000 std. cu m/hr of sour gas with 890 cu m/hr MDEA. Analytical data were measured by Sulfur Experts Inc., Calgary, at the Khangiran plant.
The amine facilities at Khangiran are designed to treat natural gas by DEA as the amine solvent. About 3 years ago, one unit was switched from DEA to MDEA and, following an analysis of the results, other units were also switched. The performance of the units using MDEA has been better than those using DEA. (Some parameters include corrosion, energy consumption, amine circulation rate, and selectivity.)
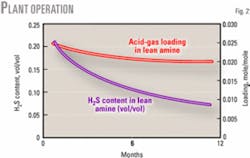
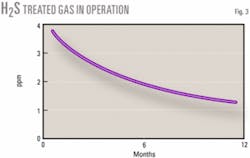
But at every unit, the initial performance of fresh MDEA at absorbing H2S in the contactors was less than desirable. Over time, however, the performance of absorber (H2S removal from the natural gas) and regenerator (acid-gas loadings in lean amine) improved and stabilized.
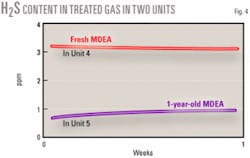
Figs. 2 and 3 show acid-gas loading, H2S content of lean MDEA, and the amount of H2S in treated gas during 1 year of operation. Comparing two unit operations at the same conditions shows that the unit performance can improve over time. One unit with fresh (Unit No. 4) and another with 1-year-old MDEA (Unit No. 5) were kept in similar circumstances for 1 week. Feed (sour gas), amine-circulation ratio, lean-amine temperature to absorbers and reboiler steam consumption were roughly equal.
Fig. 4 shows the consequences of this experiment. The 1-year-old MDEA in Unit No. 5 has been more effective than fresh MDEA in Unit No. 4. The amount of HSS was 1.11% in Unit 5 and 0.39% in Unit No. 4 solvent.
These data were measured by an on site laboratory. Amine Experts, Calgary, also tests our amine solutions and confirmed that HSSs increase over time.
Improving H2S removal
Consider a tertiary amine as an example.
When ionization of H2S in water is combined with proton acceptance by the amine, the net overall reaction is: H2S + RN ↔ RNH+ +HS-. The equilibrium constant for the reaction is K = (RNH+) (HS-)/(RN) (H2S).
It can be seen that if the concentration of the protonated form of the amine is increased, the concentration of HS- will decrease in proportion, i.e., in the regenerator, the H2S loading will drop.
This has the greatest potential impact at very low loadings because here is where the protonated amine concentration normally would be quite small (e.g., 0.001 mole/mole) but has been artificially made quite large. Consequently, there is a dramatically decreased equilibrium concentration of HS- in solution and, therefore, a dramatic decrease in solution loading with respect to H2S.
The reaction equilibrium has been shifted towards much lower H2S loadings. This can lead to a regenerated solvent with more than an order-of-magnitude lower H2S loading and, therefore, with the ability to remove H2S to much lower levels.
The effect is very pronounced at the lean end of the regenerator (especially the reboiler) where the protonated amine and HS- concentrations are already fairly small. Here, artificially enhancing the protonated amine concentration from a very small value to a relatively very large one results in a very large decrease in H2S loading of the lean solvent.
It is remarkable that there is a similar reduction in residual CO2 loadings because protonated amine is also a component in CO2 reaction equilibrium. Although tertiary amines do not react directly with CO2, they act as proton acceptors and can therefore take part in CO2 reaction equilibrium CO2 + RN + H2O ↔ RNH+ + HCO3-.
The reaction equilibrium constant is K = (RNH+) (HCO3-)/(RN) (CO2) (H2O).
Independently increasing the protonated amine concentration, especially at low loadings where the concentration of this species is normally quite small, will require a substantial decrease in the already-small HCO3- ion concentration to balance it.
References
- Weiland, Ralph H., and Sivasubramanian, M.S., “Effect of Heat-Stable Salts on Amine Absorber and Regenerator Performance,” Fall Meeting of AIChE, Austin, Nov. 7, 2004.
- “Shahid Hashemi Nejad Gas, Foaming Problems,” Amine Experts Inc., Amine System Review, Oct. 25, 2005.
The authors
Saeid Zarrabi ([email protected]) is head of utility at Shahid Hashemi Nejad (Khangiran) Gas Processing Co. with 11 years’ experience at gas treating, sulfur recovery, and utility. He holds a BS (1994) in chemical engineering from Petroleum University of Technology, Ahwaz, Iran.
Yahya Feyzi ([email protected]) is head of process operation at Shahid Hashemi Nejad (Khangiran) Gas Processing Co. with 10 years’ experience at gas treating and sulfur recovery. He holds a BS (1994) in chemical engineering from Petroleum University of Technology.
Mohammad Imanifar ([email protected]) is head of the topping plant at Shahid Hashemi Nejad (Khangiran) gas refinery with 4 years’ experience at gas treating and liquid gas distillation. He holds a BS (1998) in chemical engineering from Tehran University.