SPECIAL REPORT: Close monitoring, quick action reduce CW system problems
Stress variations in any cooling-water system bring about changes in the potential for scale, corrosion, and fouling. The ability to monitor the changing potential for these operational problems, detect upsets, and take appropriate, corrective action becomes increasingly important as the systems are pushed harder to reduce total cost of operation.
Over the past 2 years, new methods for managing open industrial cooling-water systems based on the actual stresses placed upon them have been developed and evaluated. This article discusses three applications in which variation in system stress presented the potential for scale, corrosion, and microbial fouling. Operational data will show how these stresses were managed with a comprehensive treatment, monitoring, and control strategy.
Process water
Continuous, reliable, efficient, and safe processing of clean sales gas is the primary goal of any gas processor. Cooling systems play an important role in that process, providing cooling for gas sweetening, compression, and fractionation units. Often located in areas where foulants become entrained in the cooling water, gas processing plants strive to minimize operating costs and prevent operational problems under high stress conditions.
Process water is used to heat or cool a process. Often, evaporative cooling-a process that involves recirculating the water through a cooling tower and using evaporation to cool it-provides most of the heat rejection. Obviously, as water evaporates from the process, it must be replaced. That makeup water often comes from publicly owned treatment works or a well.
The quality of makeup water sources varies. Some are more naturally scale-forming than others; some are more corrosive than others. Treating water with problematic but relatively unchanging chemical compositions-pH, conductivity, hardness, phosphate, chloride concentration, etc.-is straightforward. The water chemistry is analyzed and a treatment program put in place to prevent operational problems.
In many parts of the world, however, municipal water supplies are being severely strained as demand upon them increases. Users cannot assume they will have access in future to the same quality or quantity of water they received in the past. Their water infrastructure is aging. Their demand for water is increasing. Water-quality requirements are higher today than ever.
The end result for industrial users: higher water costs, more calls from government and concerned citizens for responsible use of natural resources and a quest for viable alternatives.
Alternative sources of makeup water exist: tertiary treated wastewater, water cascaded through other industrial processes, or water from brackish sources unacceptable for other uses. Although these sources are inexpensive and offer an environmentally friendly alternative to municipal sources, they are also highly variable.
It is not uncommon for the chemical composition of these waters to vary from scale forming to corrosive over relatively short periods of time. Microbial populations can vary over time as well. Contamination with all manner of compounds, many of which can cause serious damage to the cooling system, can occur without warning in response to causes beyond the control of the end users.
Defining system stress
For this discussion, system stress refers to any mechanical, operational, or chemical aspect of system operation that could result in an operational problem: mineral scale, corrosion, or microbial fouling.
Here are some examples of stresses placed on cooling systems:
- Variable makeup water phosphate concentration. When concentrations exceed solubility limits, calcium phosphate scaling occurs and heat-exchanger efficiency decreases.
- Variable cooling water pH. The pH varies in response to changes beyond the control of the system operators. When the pH drops, corrosion occurs, reducing equipment life.
- Variation in microbial activity. Introduction of nutrients from process streams or changes in makeup water source change the biological loading on a cooling system. Without timely addition of a biocide, microbial fouling occurs and system performance degrades rapidly.
- Operational constraints. Cost-reduction efforts have decreased the number of operators available for routine cooling-system testing and monitoring. The time between detection of a problem and proper corrective action is long enough to allow scale, corrosion, or fouling to occur.
- Operational realities. The need to take equipment off-line for periods of time influences effective biocide application, allowing biopopulations to grow.
Effective response to system stress requires constant monitoring of system parameters that reveal changing conditions. When upsets are detected, an appropriate, timely response is required to prevent a problem. Finally, information about the upset and the corrective action taken must be communicated to system users to allow them to take further corrective action, identify the root cause of the problem, or make operational changes to prevent its recurrence.
Case No. 1
A natural gas processor using cooling water to cool natural gas experienced mild-steel corrosion in several copper and steel shell-and-tube exchangers. This case study demonstrates how a comprehensive chemical treatment, monitoring, and control system can more effectively manage microbial populations by reacting to actual changes in microbial activity.
Much of the existing automation equipment was outdated and poorly maintained. This plant used conventional methods of control. It used, for example, an LMI pump for acid control of pH and conductivity as a control for blowdown. And it fed chemical continuously with another LMI pump.
In addition, no one at this plant was dedicated to cooling-system maintenance and operation.
Bacteria contamination was not uncommon. Sulfate-reducing bacteria created corrosion on the mild-steel heat exchangers. As a result, plant maintenance staff routinely repaired the exchangers. Inspection of the exchangers often revealed black slime. The high pH in the recirculating water made it corrosive to copper. Chemical dosage control was almost nonexistent.
When corrosion caused a heat exchanger failure, hydrocarbon got into the cooling water, which presented a safety issue. The cause of the problem at this plant was microbiologically induced corrosion (MIC).
Without proper treatment, bacteria form corrosive biofilms that support 99% of the bacteria in a cooling system. Most microbial monitoring systems commonly in use, such as dip slides or plate counts, measure only planktonic bacteria, those living in the bulk water, which account for the remaining 1%. Low planktonic counts often leave users with a false sense of security because they do not reveal the presence of the sessile population.
The operator adopted a novel control system to address the cause: introduction into the recirculating water of a fluorescent “bioreporter.” The bioreporter reacts with an enzyme, dehydrogenase, produced by all respiring organisms, planktonic or sessile. The reaction changes the bioreporter’s fluorescent signature.
By measuring with a modular fluorometer both species and the rates at which they changed, the control system monitored changes in the amount of actual microbial activity, adjusting biocide application as necessary to keep activity under control.
After 4 months of operation, the operator opened the exchangers for a planned maintenance shutdown, finding no microbial deposits. Since, both steel and copper corrosion rates have been below industry norms. In 2 years after adoption of the control system, no failures have occurred in any of the exchangers.
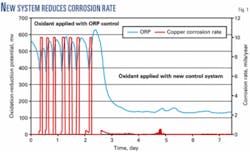
As an additional benefit, the comprehensive data-gathering capability of the control system highlighted a correlation between oxidant application and copper corrosion rates. Controlling oxidant application in response to changes in microbial activity rather than oxidation-reduction potential (ORP) reduced copper corrosion rates (Fig. 1).
Case No. 2
Effective response to upset conditions requires fast detection of the problem and prompt correction. At a petrochemical plant, calcium chloride brine contaminated the cooling system. The implication was a heightened potential for calcium carbonate scale formation and corrosion. Calcium carbonate is a tenacious, highly insulating deposit. Once formed, it can generally be removed only through acid cleaning or other expensive, time-consuming processes.
This case demonstrates how a comprehensive chemical treatment, monitoring, and control system can detect an upset and correct it before system integrity is compromised.
The plant’s cooling system was well designed. A comprehensive treatment, monitoring, and control system tracked key system parameters and was designed to take appropriate corrective action should an upset be detected.
Scale formation robs the cooling system of efficiency and can cause equipment failure. In this case, the calcium chloride brine leak increased the conductivity and chloride concentration of the recirculating water. Both of these effects increased the demand for dispersant polymer as the potential for calcium carbonate formation increased.
The chemical treatment, monitoring, and control system in place detected these chemical changes and applied more dispersant polymer to prevent deposition.
Dilution of the recirculating water through increased blowdown was another appropriate-but time-consuming-response. When plant personnel detected the upset, they increased the blowdown to reduce the calcium chloride concentration.
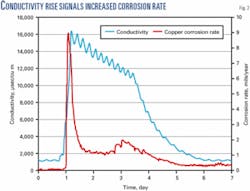
The combination of automatic corrective action by the control system and fast action by operators prevented a serious operational problem. In addition to the increased potential for scale formation, elevated chlorides increased corrosion potential. In this case, copper corrosion rates increased (Fig. 2). As blowdown reduced the chloride concentration, copper corrosion rates decreased.
The chemical treatment, monitoring, and control system in place at this facility prevented an operational problem by responding to upset conditions with an appropriate, automatic, corrective action (Fig. 3).
System operators minimized potential damage by diluting the recirculating water. The latter action could have been taken more quickly had the system alerted operators via page, email, or text message. Additional communications capability has been added to do just that.
Case No. 3
In this final case study, improved efficiency in critical heat exchangers improved control of calcium phosphate scale.
Conditions
Retubing and replacement of exchangers coupled with unnecessary downtime translated into a higher total cost of operation for this chemical plant that produces vinyl products-chlorine, caustic soda, vinyl chloride monomer, and phosphorus trichloride. Conducted with Nalco sales engineers, a review of the mechanical, chemical, and operational aspects of system operation revealed some opportunities for improvement.
This case demonstrates how a comprehensive chemical treatment, monitoring, and control system can compensate for water chemistry variation, prevent operational problems and minimize operating costs.
High temperatures (>140° F.; 60° C.) and low flow rates (0.6 fps; 0.5 m/sec) contributed to Ca3(PO4)2 scaling. The mechanical changes needed to address the high temperatures and low flows directly were not practical; operational and chemical solutions were therefore needed to address the mechanical problems.
Mineral scales such as calcium phosphate are poor thermal conductors. When critical heat exchangers foul with mineral scales, product quality, operational costs, or production rates suffer. Mineral scales restrict flow through tube bundles, reducing heat-transfer efficiency and making temperature control more difficult.
An online fluorometer controlled application of a polyphosphate-based corrosion inhibition program. A very accurate method, fluorescence-based control ensured the target dose of inhibitor was always present in the recirculating water. Good corrosion inhibition had been achieved historically.
Polyphosphate inhibited corrosion at the cathode; orthophosphate inhibited corrosion at the anode. The program worked well when proper concentrations of both species were maintained. Results suffered when the balance was upset, either through high temperatures, low flows, variations in the amount of orthophosphate present in the makeup water, or other uncontrollable factors. In the presence of too much orthophosphate, Ca3(PO4)2 scale formed. When orthophosphate concentrations were too low, corrosion occurred.
Loss of heat-transfer efficiency through Ca3(PO4)2 scaling represented a greater concern than corrosion. As a result, the operator maintained cooling-water pH at 6.8 to reduce potential for scale formation. The mineral solubility models employed to make this decision were based on discreet chemical conditions, essentially snapshots.
Without the ability to model the variability in the system, control decisions were based on worst-case scaling conditions. When orthophosphate concentrations dropped, no risk of scale formation existed, but corrosion rates increased. A more dynamic modeling tool was needed to understand the stresses placed on this system more completely.
The facility also maintained relatively high halogen concentrations that contributed to higher copper corrosion rates and degradation of the copper corrosion inhibitor. High halogen concentrations degraded tolyltriazole, the copper corrosion inhibitor in use. The result was high copper corrosion rates.
Copper deposition also contributed to corrosion of mild-steel heat exchangers. High chlorine residuals were necessary to comply with country-specific legionella control regulations. The facility had used both shock dosing and continuous chlorination at times.
Approach
To address these problems, the operator adopted a program that consisted of new chemical treatment combined with a monitoring and control system. This program continuously measured key parameters related to system stress, detecting upset conditions and taking appropriate, automatic corrective action. It then communicated with users, documenting what occurred, when, and what action was taken.
Uncontrolled reversion of the polyphosphate to orthophosphate was a key contributor to Ca3(PO4)2 scale formation. The new program addressed the issue both chemically and through improved control.
Phosphino succinic oligomer (PSO) is a corrosion inhibitor that does not revert to orthophosphate as do traditional polyphosphates. By eliminating the orthophosphate contributed through reversion, the control automation could adjust system operation based on only one source of phosphate variation: the changing concentrations in the makeup water. Eliminating one source of variability made both scale and corrosion control more successful (Fig. 4).
The monitoring and control technology used in this system combines fluorescence-based monitoring of an inert fluorescent material with an innovative chemical “tag” incorporated into the dispersant polymer backbone. The chemical “tag” responds to stress just as the polymer does. By continuously comparing the inert signal and the “tag” and the rates at which they change, the system can adjust blowdown and chemical feed to prevent scale formation. During periods of high scale-forming stress, more dispersant polymer was applied.
As the stress abated, less was applied, preventing Ca3(PO4)2 scale formation and minimizing operational costs. Combining such innovative chemistries as PSO and “tagged” polymers with advanced control techniques provided this facility good return on investment.
Benzotriazole, a much more halogen-resistant azole, replaced tolyltriazole as the copper corrosion inhibitor and eliminated copper deposition through better copper corrosion inhibition.
In addition to the changes to the chemical program and control strategies, the cooling water pH was increased to 7.2. Computer modeling predicted good results-low corrosion rates and reduced potential for scale formation-at that pH when coupled with PSO and better control. This reduced acid feed and made the water chemistry less corrosive.
Results
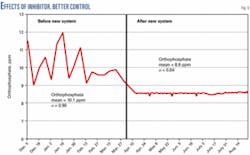
The combination of a more reversion-resistant corrosion inhibitor and better control translated into lower corrosion rates and better scale control at this facility. Fig. 5 shows the reduction in overall orthophosphate concentrations and variability. Visual inspections of heat exchangers and corrosion coupons verified the improvement (Fig. 6).
null
Since the DPD chlorine analyzer was already installed, its signal output was combined with the other data collected by the new control system. The trend of free chlorine measured by the analyzer correlates well with the ORP measurement taken by the controller.
Plant engineers estimate the annual total cost of operation reduction at their facility to be $168,000, including $52,000 in heat exchanger cleaning, retubing, and repair costs.
Acknowledgment
The author thanks colleague Andrea Marek for her contributions in preparation and presentation of this article.
The author
Daniel M. Cicero is a senior product manager in Nalco’s Product Lifecycle Management Group. He has been with Nalco for more than 15 years in a variety of sales, marketing, new-product development, and product-line management roles. He is currently brand manager for Nalco’s 3D TRASAR technology.