Egyptian gas plant employs absorbents for Hg removal
Initial mercury measurements in 2000 for gas upstream of Khalda Petroleum Co.’s Salam gas plant in Egypt’s Western Desert showed mercury content between 75 and 175 μg/std. cu m. Concern for the plant’s downstream aluminum heat exchangers led the company to decide, therefore, to install mercury-removal equipment.
To avoid mercury condensation under pipeline conditions, mercury should be removed from sales gases to less than 20 μg/std. cu m.1
The company evaluated two alternatives-UOP’s HgSIV adsorbents and Puraspec’s 1156 absorbents-for removing mercury from natural gas.
This article will review the corrosion mechanism of mercury and aluminum, the analysis techniques for mercury in natural gas, and the mercury-removal techniques from natural gas and present the process design, field analysis procedures, and performance of Salam mercury-removal unit.
Mercury
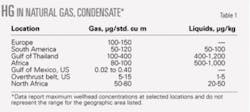
Mercury occurs naturally in trace quantities in air and natural gas. Although difficult to generalize, mercury concentrations in air typically range between 0.001 and 10 μg/std. cu m.2-5 Some authors report that natural gas typically contains mercury concentrations between 1-200 μg/std. cu m.6 7 Table 1 shows that hydrocarbons from a few geographic locations contain mercury at concentrations exceeding 100 μg/std. cu m for gas and 100 μg/kg for gas condensate (C3-C6).8
Low levels of mercury are frequently found in low-sulfur-containing hydrocarbon gases and liquids such as those produced in the North Sea. In the Netherlands, the mercury content of natural gas varies from “nondemonstrable” to about 300 μg/std. cu m, where 250 μg/std. cu m is considered a high concentration.9
Implications of the effect of mercury in natural gas were not reported until 1973, when a catastrophic failure of aluminum heat exchangers occurred at Skikda LNG plant in Algeria (OGJ, Sept. 15, 1975, p. 192). It was found that a combination of mercury and water temperatures around 0.0º C. caused corrosion in the aluminum tubes (constructed of aluminum alloy 6061). This discovery prompted several research studies into this phenomenon.
After the Skikda failure, a study of the Groningen field in Holland revealed similar corrosion in the gas gathering system. CO2 was initially thought to be the cause,10 but later investigations pointed to mercury, with concentrations ranging from 0.001 to as high as 180 μg/std. cu m.11 Although the concentration of mercury in a given natural gas may be considered extremely low, Audeh observed that its effect is “cumulative as it amalgamates.”12
Elemental mercury forms an amalgam with the surface layer of the metal it contacts. With aluminum, the amalgam is much weaker than the metal itself.11 The mercury-aluminum amalgam process removes the tightly adhering aluminum oxide layer, which then allows aluminum corrosion to occur.
It can be removed chemically or mechanically and is catalyzed by the presence of an aqueous electrolyte. The mercury-aluminum amalgamation generally does not occur as a direct chemical reaction because the base-metal aluminum is usually protected by the oxide film. It is formed in continuous chemical reactions.
The aqueous corrosion cell forms aluminum hydroxide and gaseous hydrogen through the following reactions:2
These reactions leave the previously amalgamated mercury free to form additional amalgam with the base metal in a continuous corrosion process.
In experiments on various Al-Hg systems, Phannensteil reported having ions present in the condensed water was necessary for corrosion to occur and that it was unnecessary for the protective aluminum oxide film to be removed to initiate the reaction.11 This contradicts all other studies on this subject.
Analysis, removal techniques
Keeping the sales gas and emission streams to appropriate specifications requires reliable methods of analyzing the mercury content of gas streams. Toxicology and environmental studies have been involved in development of several methods for detecting mercury in natural gas.
Table 2 summarizes the laboratory methods and depicts measurements of a finite limit of mercury mass.2 7 Table 3 summarizes several techniques for removing mercury from the gas stream. Most of these methods have limitations that detract from their applicability to natural gas processing.
The basic requirement for successful, economical mercury removal is that the removal medium be capable of reducing mercury concentrations to extremely low levels. The medium must have a high capacity for an active bonding to mercury and must retain the mercury in a form that can be disposed of.
It must be effective at the operating pressures and temperatures of the natural gas stream, have reasonable investment and operating costs, and be readily available.
Salam gas plant
Khalda Western Desert gas development project at Salam is 70 km from Matrouh. The plant processes gas condensate from Salam field, Qasr field, South Umbarka, and an oil plant’s associated gas. The project produces about 200 MMscfd of export gas at an export pressure of 101 bara and 9,000 stk. tank bbl of condensate.
The sales gas is designed to have a maximum CO2 content of 3%, a maximum H2S content of 4 ppm (vol), a gross heating value greater than 1,040 btu/std. cu ft, a water dewpoint of less than 0.0º C. at 71 bara, and a cricondentherm of 5º C. The condensate is designed to have a maximum of 11-psi rvp.
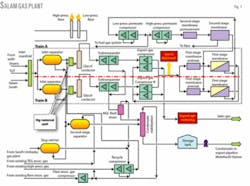
Fig. 1, a diagram of the Salam gas plant, shows gas from the wells flows into two parallel trains.
First it enters a three-phase separator where the main water-condensate-gas separation takes place. Gas from the three-phase separator goes to the mercury-removal unit. Then it flows to the glycol contactors to remove water from the gas to avoid hydrate formation and to achieve water-content specifications.
Gas is then diverted to the dewpointing package, whose function is to separate entrained traces of condensate and heavier hydrocarbons that condense as liquids from the gas at lower temperatures. This step is to achieve hydrocarbon dewpoint specifications with the turbo-expanders.
After dewpointing, the gas enters the gas-sweetening system (two-stage membrane package) to reduce CO2 in the export gas. The final step is to export the gas via the export compressors.
Condensate collected from the various processing steps moves to stabilization before being stored in the three storage tanks. The stabilizer tower removes the light hydrocarbons to avoid release in the tanks and to achieve the rvp specification. Condensate is then shipped from the storage tanks via shipping pumps to El-Hamra.
Removal options
Initial mercury measurements for gas upstream of the Salam gas plant 5 years ago showed that the mercury content varied between 75 and 175 μg/std. cu m. This high content of mercury and its compounds had to be removed from the gas to protect downstream aluminum heat exchangers. To avoid mercury condensation under pipeline conditions, mercury should be removed from sales gases to less than 20 μg/std. cu m.1
Operations at Khalda Petroleum evaluated two alternatives (UOP HgSIV adsorbents and Puraspec 1156 absorbents) for removing mercury from natural gas. Following are the characteristics of both catalysts.
HgSIV adsorbents were developed for effective mercury-removal adsorption units.
These adsorbents are molecular-sieve products that contain silver on the surface of the pellet or bead. Mercury from the process fluid (gas or liquid) amalgamates with the silver and yields a mercury-free dry process fluid. Mercury is regenerated from the HgSIV adsorbents with conventional gas dryer techniques.
Physically, HgSIV adsorbents are available as beads or pellets and are loaded into an adsorption vessel just as are conventional molecular sieves.
UOP has installed HgSIV adsorbents units in several gas processing plants in the Far East, Middle East, Africa, South America, and the US. The feed-gas mercury content for these adsorbents units ranges from 25 to 50 μg/std. cu m. The mercury level in the treated natural gas is about 0.01 μg/std. cu m.
Puraspec 1156 absorbents (pre-activated; sulfided) are by their nature active material. They consist of a high porosity, spherical mixed-sulfide absorbent of different diameters. The absorbents are loaded into a mercury-removal vessel where the mercury is irreversibly bound to them and cannot, therefore, affect downstream process equipment.
These absorbents remove small quantities of contaminants from a wide variety of liquid and gas streams, with high efficiency and effectiveness. The process is easy to operate and only requires periodic analyses to check performance. The absorbent unit is designed to remove mercury from an average inlet concentration of about 200 μg/std. cu m to about 0.1 μg/std. cu m.
The mercury-removal reaction by these absorbents appears below. Mercury vapor reacts with the metal sulfide to form a stable mercuric sulfide:
The reactive metal (mercury) is incorporated in an inorganic support and the absorbent is supplied with the reactive sulfide present or formed in situ by reaction with H2S in the hydrocarbon to be treated.
The absorbents have other advantages:
- They can be used for wet and dry gases.
- They retain organo-mercury compounds.
- There is no risk of loss of sulfur by sublimation or dissolution. All sulfur species are securely bound as inorganic sulfides.
- The economic evaluation indicates that Puraspec 1156 is a cost-effective catalyst with a bed life of 5 years.
Technical evaluation
Operations at Khalda Petroleum evaluated UOP’s HgSIV adsorbents and Puraspec’s 1156 absorbents for removing mercury from the feed gas upstream of the Salam plant. Table 4 presents a summary of this evaluation.
Table 4 makes clear that UOP HgSIV adsorbents can be used to treat gas with mercury contents of 50 μg/std. cu m, which is much lower than the mercury contents of the feed gas to the Salam plant (175 μg/std. cu m).
Puraspec 1156 absorbent, however, is designed to remove mercury from gas with an average inlet concentration of about 200 μg/std. cu m, which is higher than the mercury content of gas to the Salam plant. That means Puraspec 1156 absorbent can be used to treat the feed gas, while UOP HgSIV adsorbent is not suitable for treating the gas.
Table 4 shows also that both catalysts are technologies proven in many applications: Both catalysts can be used to treat gas to levels below the acceptable limit for pipeline specifications. The economic evaluation for both technologies indicated, however, that Puraspec 1156 is a cost-effective catalyst for bed life of 5 years.
Based on the previously mentioned technical and economical evaluation, Khalda Petroleum decided to use Puraspec’s absorbent for removing mercury from the feed gas upstream Salam gas plant.
Mercury removal
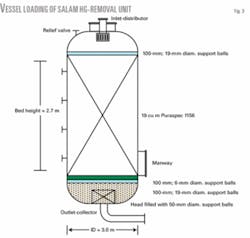
Fig. 2 shows the mercury-removal unit at the Salam gas processing plant. The mercury-removal unit is loaded with 19 tons of the catalyst Puraspec absorbent. The catalyst consists of a high porosity, spherical mixed-sulfide absorbent of different diameters as shown in Fig. 3.
null
Before the gas is diverted to the mercury-removal unit, it goes to filter-coalescers to remove the liquid carryover and particulates. Then the gas stream flows through the mercury-removal unit.
The mercury is irreversibly bound to the catalyst and therefore cannot affect downstream process equipment. Downstream of the mercury-removal units are cartridge filters. These protect the glycol units from any particulate carryover from the mercury-removal unit. All these units are skid mounted one skid per train.
Field analysis
The mercury content of the feed and the exit streams from the Salam mercury-removal unit were monitored regularly to ensure the reactor was performing satisfactorily. Mercury analyses were performed at the site with the atomic fluorescence method. The analytical equipment consists of an atomic fluorescence detector for mercury measurements and an instrument for vaporizing any hydrocarbon liquid samples.
The principle of the mercury-monitoring system is the collection of mercury in a trap at process temperatures. Dual amalgamation, thermal desorption at 800º C., and analysis by atomic fluorescence spectroscopy determine the level of mercury present in a hydrocarbon feed. Samples may be either gaseous or volatile liquids.
The principle of operation for gas samples is to pass a known volume of sample gas (measured by a rotary gas meter) through a trapping medium that concentrates the mercury species. The tube is then placed in the mercury analyzer, where it is heated to 800° C. in a flow of argon.
Under these conditions, the mercury on the trap is desorbed from the adsorbent as elemental mercury vapor and swept to a secondary fixed adsorber in the analyzer.
Subsequent heating of this tube to 800° C. in a flow of argon again desorbs the mercury, this time to an atomic fluorescence detector within the instrument. The response from the detector is proportional to the total mercury present in the sample in pico-grams (10-12 gm). The result is then reported as weight of mercury per unit volume of gas.
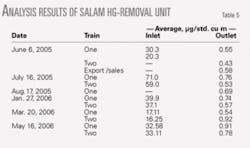
Unit performance
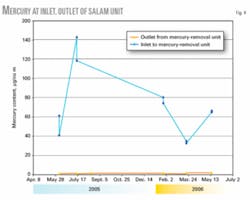
The mercury contents of the gas at the inlet and outlet of Salam mercury-removal unit (Trains 1 and 2) have been monitored since June 2005. Fig. 4 and Table 5 show some measurements carried out and analyzed. The mercury content of the gas at the inlet of the Salam mercury-removal unit has been fluctuating, with inconsistent values ranging between 17 and 71 μg/std. cu m. The mercury content of the gas at the outlet of the unit ranged between 0.53 and 0.92 μg/std. cu m during the sampling dates.
null
It is clear from these results that the mercury content of the gas at the outlet of the unit was small and almost constant (ranging between 0.53 and 0.92 μg/std. cu m), regardless of the mercury content of the gas at the inlet of the mercury-removal unit.
Results for Qasr gas
Qasr gas is produced from six wells in the Western Egyptian desert and contains some mercury. Qasr production is collected at a common manifold (Shams manifold) then diverted into three different processing plants: Salam, Obaiyed, and Tarek.
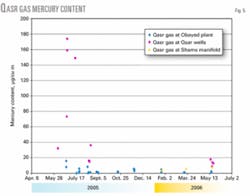
The mercury content for Qasr gas has been monitored regularly at three locations: Qasr wells, Obayed plant, and Shams manifold. Fig. 5 presents the mercury-content analysis at these sampling locations from June 2005 to May 2006. This figure shows that the mercury content of gas at Qasr wells was very high early in production, while recently it has been lower.
Fig. 5 also makes clear that mercury contents of the gas at the Obayed gas plant and Shams manifold are lower than at Qasr wells. That may be related to the ability of the mercury to precipitate on the internal surface of the pipeline.
Bingham states that as mercury enters gathering system pipelines with gas, it is reduced because of chemisorptions onto steel pipe walls.2 Leeper suggests the following reactions as driving this reduction:10
and
Trace quantities of H2S are the catalyst for the reaction of mercury with iron oxide from the pipe. The mercury-sulfide precipitates and is adsorbed onto the pipe wall. Grotewold reports that for 110-km pipeline, mercury content decreased to 20 μg/std. cu m from about 50 μg/std. cu m.13 This reduction is influenced by pipe wall roughness and adhesive forces.
Acknowledgment
The authors thank Khalda Petroleum Co. for permission to publish this article.
References
- Mussig, S., and Rothmann, B., “Mercury in Natural Gas-Problems and Technical Solutions for its removal,” SPE Paper 38088, presented at the 1997 SPE Asia Pacific Oil and Gas Conference, Kuala Lumpur, Malaysia, Apr. 14-16, 1997.
- Bingham, M.D., “Field Detection and Implications of mercury in Natural Gas,” SPE Production Engineering, May 1990 (Vol. 5), No. 2, pp. 120-24.
- Muchlis, M., “Analytical Methods For Determining Small Quantities of mercury in Natural Gas,” Proceedings, Annual Convention of the Indonesian Petroleum Association, Jakarta, May 25-27, 1981, pp. 401-421.
- Ohkawa, T., et al., “Mercury Analysis in Ambient Air by Means of Thin Gold Film Resistors,” Eisei Kagaku J. Hyglenic Chemistry, 1976, pp. 11-19.
- Dumarcy, R., Dams, R., and Hoste, J. “Comparison of the collection and Desorption Efficiency of Activated Charcoal, Silver, and Gold for the Determination of Vapor-Phase Atmospheric mercury,” Anal. Chem., November 1985 (Vol. 57, No.13), pp. 2638-2643.
- Haselden, G.G., “The Challenge of LNG in the 1980’s,” Mech. Eng., March 1981 (Vol. 103), pp. 46-53.
- Bodle, W.W., Attari, A., and Serauskas, R.: “Considerations for mercury in LNG Operation,” Inst. of Gas Technology Intl. Conference on LNG, Kyoto, Apr. 7-14, 1980.
- Mark Wilhelm, S., and McArthur, A., “Removal and Treatment of mercury Contamination at Gas Processing Facilities,” SPE 29721, SPE/EPA Exploration & Production Environmental Conference, Houston, Mar. 27-29, 1995.
- Gijselman, P.B., “Presence of mercury in Natural Gas: An Occupational Health Programme,” SPE 23200, First International Conference on Health, Safety and Environment, The Hague, Nov. 10-14, 1991.
- Leeper, J.E., “Mercury-LNG’s problem,” Hydrocarbon Processing, November 1980, pp. 237-40.
- Phannenstiel, L. L., McKinley, C., and Sorensen, J.S.: “Mercury in Natural Gas,” Paper PAP76-T-12 Presented at the 1976 American Gas Association, Operation Section Transmission Conference, Las Vegas, May 3-5.
- Audeh, C. A.: “Process for Absorbing Mercury from Natural Gas,” U.S. Patent No. 4,717,399 (Jan. 1988).
- Grotewold, G., Fuhberg, H.D., and Philipp, W.: “Production and Processing of Nitrogen-Rich Natural Gases from Reservoirs in the NE Part of the Federal Republic of Germany,” Proc., 10th World Pet. Cong., Bucharest (1979) 4,47-54.
The authors
Mahmoud Abu El Ela ([email protected]) is an assistant professor in the Petroleum Engineering Department, Cairo University, and also works as a petroleum process consulting engineer at Khalda Petroleum Co. He previously worked as a research engineer at Woodside Research Foundation, Curtin University of Technology-Australia. Abu El Ela holds a BSc and MSc in petroleum engineering from Cairo University (Egypt) and a PhD from Curtin University of Technology (Australia). He is a member of the Egyptian Engineers Syndicate and SPE.
Ismail Mahgoub is chairman of Khalda Petroleum. He previously served as chairman for Petrozeit Co., operations general manager for Khalda Petroleum, assistant general manager for Wepco Petroleum Co., and associate professor for Cairo University. Mahgoud holds a BSc in petroleum engineering from Cairo University (Egypt) and a PhD from Institut National Polytechnique de Lorraine, Nancy, France. He is a member in the Egyptian Engineers Syndicate and SPE.
Mostafa Nabawi is a general manager in the gas operation department at Khalda Petroleum. He previously worked as assistant general manager in the gas operation department at Khalda Petroleum. Nabawi holds a BSc in mechanical engineering from Assuit University, Egypt. He is a member in the Egyptian Engineers Syndicate and SPE.
Mohamed Abdel Aziem is an assistant general manager in the gas operation department at Khalda Petroleum. He holds a BSc in petroleum engineering from El Azhar University, Cairo. He is a member in the Egyptian Engineers Syndicate and SPS.