Correlation pegs adsorption limits of activated carbon for chlorinated petchems in water
This article presents a correlation that accurately estimates the adsorption capacity of activated carbon for removing chlorinated petrochemicals in water.
Results use the Freundlich equation for adsorption capacity as a function of the compound concentration in aqueous liquid. The correlation constants are presented in a tabular format, which makes for rapid engineering use. Correlation and experimental results agree favorably.
A previous article (OGJ, May 5, 2003, p. 80) developed a correlation for the adsorption capacity of activated carbon for alkyl benzenes in water.
Background
Physical and thermodynamic property data for chlorinated petrochemicals are especially helpful to engineers and scientists in industry. In particular, capacity data for adsorption of chlorinated petrochemicals on activated carbon are increasingly important in engineering and environmental studies because of more stringent environmental regulations involving water.
The results in this article are suitable for engineering and environmental studies. Capacity data from the correlation, for example, are useful in the engineering design of carbon-adsorption systems to remove chlorinated petrochemicals from water.
Correlation
The correlation for adsorption capacity of activated carbon (Calgon Carbon Corp.'s Filtrasorb 400) as a function of the compound concentration in water is based on the Freundlich equation (see Equations box).
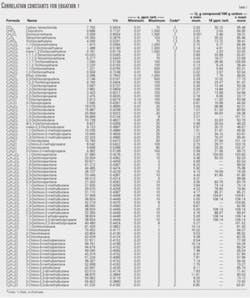
Table 1 also shows the correlation constants, K and 1/n, for various chlorinated petrochemicals. The tabulation is based on both experimental and estimated values. Table 1 is arranged by carbon number to make it easy to use for quickly locating data using the chemical formula.
Table 1 shows the adsorption capacity at concentrations of x minimum, 10 ppm (wt), and x maximum in water.
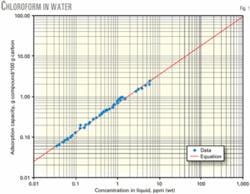
Fig. 1 shows a comparison of correlation and experimental data for a representative compound, chloroform. Adsorption capacity is at typical conditions encountered in water pollution control. The graph shows a favorable agreement between correlation and experimental data.
Estimation equation
In preparing the correlation, we conducted a literature search to identify source publications relative to experimental data and property values for estimates.1-5 We used appropriate data to create a database of adsorption-capacity values at different concentrations for which experimental data are available. The database also served as a basis to check the accuracy of the correlation.
After data collection, we estimated adsorption capacity for the remaining compounds. For chlorinated petrochemicals, we used Equation 2 (developed from the data of Speth3) for estimates.
For Equation 2, the data for density, liquid molar volume, solubility in water, and refractive index are from data compilations by Yaws.4 5
In general, the literature only contains limited experimental data for adsorption capacity of activated carbon for chlorinated petrochemicals in aqueous liquid at concentrations of several hundred parts per million. Because of this, the estimates in the tabulation are rough approximations.
Also, different lots of carbon, sources of water, and concentration ranges complicate the adsorption capacity data.2 If initial feasibility studies using the tabulated values are favorable, we recommend a follow-up experimental determination of equilibrium adsorption capacity.
Example calculation
Water from an industrial process contains 10 ppm (wt) of tetrachloroethylene (C2Cl4). Estimate the adsorption capacity of activated carbon for removing the compound from water.
Substitution of the coefficients from Table 1 and concentration into the correlation equation yields:
log10 Q = log10 (14.35) + 0.3875 log10(10) = 1.5443
Q = 101.5443
Q = 35.02 g tetrachloroethylene/100 g carbon.
References
1. Sontheimer, H., Crittenden, J.C., and Summers, R.S., Activated carbon for water treatment, 2nd Edition, Deutsche Vereinigung des Gas und Wasserfaches-Forschungsstelle, Engler-Bunte-Institut, University of Karlsruhe, Germany, 1988.
2. Speth, T.F., and Miller, J.R., Journal AWWA, Vol. 72, February 1990.
3. Speth, T.F., master's thesis, Michigan Technological University, Houghton, Mich., 1986.
4. Yaws, C.L., Chemical Properties Handbook, McGraw-Hill Co., New York, 1999.
5. Yaws, C.L., Yaws Handbook of Thermodynamic and Physical Properties of Chemical Compounds, electronic edition, www.knovel.com, Knovel Corp., Norwich, NY, 2003.
The authors
Carl L. Yaws is professor of chemical engineering at Lamar University, Beaumont, Tex. His research interests include technology development, thermodynamic and transport property data, environmental engineering, and process simulation. Yaws holds BS in chemical engineering from Texas A&I University, Kingsville, and an MS and PhD in chemical engineering from the University of Houston. He is a registered professional engineer in Texas.
Prasad K. Narasimhan is graduate student in chemical engineering at Lamar University. His research interests include thermodynamics, environmental engineering, and process simulation. He holds a BS in chemical engineering from Siddaganga Institute of Technology, India.
Ralph W. Pike is Paul M. Horton Professor of Chemical Engineering and the director of the Minerals Processing Institute at Louisiana State University, Baton Rouge. His research interests include process optimization, fluid dynamics, reactor design, ecological systems, and pollution prevention. Pike holds a BS and PhD from Georgia Institute of Technology, Atlanta. He is a registered professional engineer in Louisiana and Texas.