A systematic study conducted in Lukoil Bulgaria Ltd.'s Neftochim Bourgas FCC unit quantified the influence of unit operating variables on FCC gasoline specifications.
We specifically studied the effect of FCC feed hydrodesulfurization (HDS) severity, operating conditions, and the characteristics of two modern FCC catalysts (an octane-barrel catalyst and a maximum-octane catalyst) on the properties of the resulting FCC gasoline—specifically gasoline sulfur, hydrocarbon distribution, and octane.
The study showed that, whereas feed hydrotreating severity significantly affects gasoline sulfur, it is the FCC catalyst properties that have the biggest impact on gasoline olefinicity and octane.
We evaluated economic results for two different catalysts in both the FCC reactor and FCC feed pretreating section.
The study showed that replacing a nickel-molybdenum (Ni-Mo) catalyst with a cobalt-molybdenum (Co-Mo) catalyst in the FCC feed pretreating section resulted in a margin increase of $366,160/year. This is due to 5.2% more production of low-sulfur (<350 ppm) diesel and less RON 91 gasoline produced.
Changing the octane-barrel FCC catalyst with a maximum-octane catalyst resulted in a margin increase of $105,225/year. This was due to 17.6% more RON 95 unleaded gasoline produced at the expense of less RON 92 unleaded gasoline.
FCC operations
Tighter gasoline quality specifications are forcing refiners to produce gasoline with extremely low sulfur and olefins content. FCC gasoline typically contributes about 40% of the finished gasoline volume and is responsible for about 90% of the sulfur and olefins in the finished gasoline pool.1-3
To meet the specifications for gasoline sulfur and olefins, therefore, refiners are focusing on FCC unit operations. Refiners currently have several process options to lower FCC gasoline sulfur.
Feed hydrotreating produces low-sulfur transportation fuels and also reduces SOx emissions from the FCC regenerator.4-6 Feed hydrotreating capacity, however, is expensive and many refiners choose to desulfurize the FCC gasoline because it is less capital intensive.
Hydrotreating FCC gasoline, however, has its own problems, including a loss of octane.
There are several process options available for FCC gasoline sulfur reduction and each is designed to minimize the impact on gasoline octane.7-13
Specially designed catalysts and catalyst additives have reduced gasoline sulfur by as much as 50% in commercial FCC units without degrading other product yields (OGJ, Feb. 12, 2001, p. 54).14, 15
No current catalyst or additive technology alone, however, can achieve the levels of sulfur reduction needed to meet the tightest quality specifications.
A greater equilibrium unit cell size of an FCC catalyst reduces gasoline olefinicity.
More-sophisticated FCC catalysts, however, contain zeolites with high hydrogen-transfer properties and also contain specially designed matrix technology that helps further reduce gasoline olefins.
Mild hydrotreating is also an option to reduce gasoline olefins although the octane penalty can be significant.
Bourgas FCC unit
The FCC unit in Lukoil Neftochim's Bourgas refinery is a Grozni design. It started up in 1982.
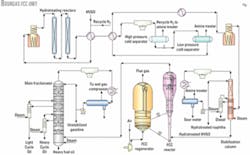
The unit consists of an FCC feed hydrotreating section, FCC reactor-regenerator and fractionation section, vapor-recovery section, and mercaptan removal from the FCC gasoline.
Fig. 1 shows a diagram of the hydrotreating, reaction and fractionation sections in the FCC unit.
In 1992, the FCC reactor was revamped and the catalyst-disengaging device replaced with a ballistic separator. This reconstruction resulted in a shorter catalyst-oil contact time in the riser and an increase of valuable product yields due to reduced secondary cracking.
In 1998, the FCC feed injection system, which was a "shower head" type, was replaced with ATOMAX feed nozzles.
Feed hydrotreating
The typical FCC feedstock is a heavy vacuum gas oil (HVGO), produced from a mixture of Russian export blend crude, reduced crude, vacuum gas oil, and naphtha.
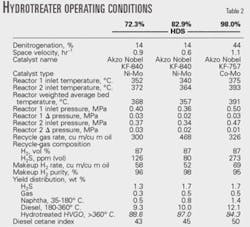
Table 1 shows the HVGO and hydrotreated HVGO properties produced at different HDS severities.
Table 2 summarizes the operating conditions in the hydrotreating reactors and the yield distribution in the hydrotreating section.
Hydrotreating the HVGO reduces mainly sulfur, metals, and concarbon.
At low reactor pressure, the degree of denitrogenation and aromatic hydrogenation is low. Increasing the pressure to 0.5 MPa (72.5 psi) from 0.36 MPa increases the extent of denitrogenation to 44% from 14%, the hydrogen content in the hydrotreated HVGO to 12.9% from 12.4%, and the diesel cetane index to 50 from 43.
This is despite the fact that the catalyst operating at this pressure is a Co-Mo catalyst. Co-Mo hydrotreating catalysts have lower hydrogenation activity compared to Ni-Mo catalysts,18 and new systems are being commercialized that take advantage of the benefits of combining Ni-Mo and Co-Mo catalyst technologies for greater HDS and hydrodenitrogenation.19
About 5% of the gas oil sulfur compounds produce gasoline-range sulfur products in the FCC gasoline (OGJ, June 7, 1999, p. 55).20 21
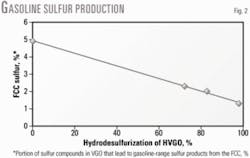
Fig. 2 shows the relationship between HVGO feed HDS severity and the percentage of sulfur species in the FCC feed that produce gasoline-range sulfur products in catalytic cracking.
Data indicate that increasing the FCC feed HDS severity decreases the percentage of sulfur compounds in the vacuum gas oil that produce gasoline-range sulfur products in the FCC unit.
For example, during cracking of a virgin HVGO, 4.9% of its sulfur components end up as gasoline-range sulfur products compared with 2.3% and 1.3% when FCC feed HDS severity increases to 72.3% and 98%, respectively. A higher FCC feed HDS severity allows removal of these sulfur compounds.
The data also show that every 100-ppm increase in the Bourgas FCC feed raises sulfur by 3 ppm in the FCC gasoline cut with a final boiling point of 215° C.
Our analysis of the sulfur species in the FCC gasoline obtained from cracking virgin HVGO and hydrotreated HVGO at different HDS severities shows that they consist of thiophene and its alkyl derivatives (Table 3).
Gasoline produced from virgin HVGO also contains some thiophenol. The distribution of sulfur compounds is approximately the same for all the FCC gasoline samples, with methythiophenes and ethylthiophenes having the highest concentrations.
Increasing feed HDS severity reduced the C3 and C4 thiophenes; at HDS severities greater than 82.9%, the FCC gasoline is essentially free from these thiophenes as well as tetrahydrothiophene.
Reducing FCC gasoline FBP to less than 221° C. provides an additional opportunity to decrease sulfur content by about one third. For example, this FBP reduction produced gasoline with 8-ppm sulfur from deeply hydrotreated feedstock (98% HDS). This value meets most severe requirements for sulfur content.
The cut points reported here are based on perfect cuts; in commercial operations, the gasoline end point should be well below 221° C. to significantly reduce gasoline sulfur by removing benzothiophene.
Thiophene and its alkyl derivatives have significantly higher HDS reactivity compared to polycyclic sulfur-containing components that are prevalent in vacuum gas oil.22
This explains why there is a substantial reduction in feed sulfur species that end up in the FCC gasoline after catalytic cracking when feed HDS severity increases.
The substitution of Ni-Mo catalyst with a Co-Mo catalyst, combined with some FCC feed hydrotreater operating modifications, can help produce gasoline with sulfur content of less than 10 ppm.
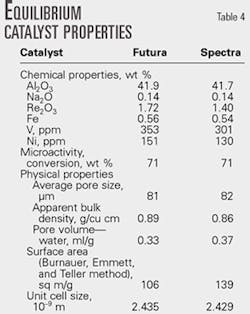
Catalyst properties, operating conditions
The octane number of cracked gasoline depends on the feed type, catalyst properties, reactor temperature, and conversion (OGJ, Aug. 5, 1985, p. 91; OGJ, Aug. 22, 1988, p. 51).
We studied two types of feedstocks: HVGO and hydrotreated HVGO. We evaluated two types of FCC catalyst—an octane-barrel and maximum-octane catalyst—for their impact on gasoline octane.
The octane-barrel catalyst was from the Futura catalyst products series and the maximum-octane catalyst was from the Spectra catalyst products. Grace Davison, Worms, Germany, supplied both catalysts.
Catalyst features and properties are described elsewhere.23 24
Table 4 lists the catalyst properties used in the Lukoil Neftochim Bourgas FCC unit.
We varied the unit conversion by 72.0-81.5% by changing the catalyst: oil ratio 5.5-8.5 and by changing the reactor temperature 500-533° C.
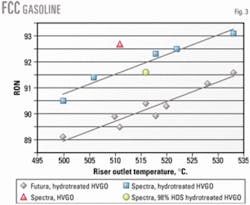
Fig. 3 shows the impact of feed type, catalyst type, and reactor temperature on FCC gasoline octane. For each 10° C. increase in reactor temperature, the FCC gasoline octane increases by 1 number with the hydrotreated HVGO feed.
The data indicate that when hydrotreated HVGO is processed, the octane-maximizing catalyst gives gasoline with about 2 octane numbers greater octane than the other catalyst. HVGO feed generally produces a gasoline with octane values about 1 number greater than the gasoline produced from the hydrotreated HVGO.
Fig. 3 also shows that, compared with a change in catalyst properties (i.e., hydrogen transfer activity), deep HDS of HVGO does not have a significant impact on FCC gasoline octane.
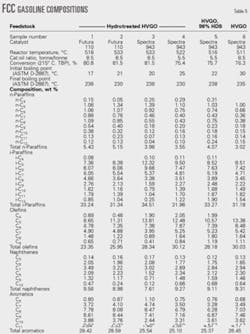
We analyzed six FCC gasoline samples to understand better the role of each variable on gasoline octane.
Table 5 shows the sampling conditions and relevant hydrocarbon compositions.
A comparison of Samples 1 and 2 and of Samples 4 and 5 best illustrates the influence of reactor temperature on hydrocarbon composition of FCC gasoline.
Cracking hydrotreated HVGO on the octane-barrel catalyst at identical catalyst:oil ratios and different reactor temperatures produced Samples 1 and 2. Samples 4 and 5 resulted from cracking hydrotreated HVGO on the maximum-octane catalyst at identical catalyst:oil ratios and different reactor temperatures.
The temperature increase at a constant catalyst:oil increases the ratio of b-scission cracking to hydrogen transfer.
This results in higher content of lower-molecular-weight hydrocarbons and higher gasoline olefinicity. Those two factors positively affect the octane number of cracked gasoline.25 26
Gasoline olefinicity, for example, expressed as the percentage of olefins vs. olefins plus paraffins in Sample 1 is 37.6% while Sample 2 is 41.6 %; in sample 5 it is 43.0% and in Sample 4 it is 45.9%.
The ratio of aliphatic hydrocarbons (C5 + C6 + C7)/(C8 + C9 + C10 + C11 + C12) in Sample 1 is 2.6 while in Sample 2 it is 3.5. Similarly, this ratio in Sample 5 is 2.5 and in Sample 4 it is 3.1.
The aromatics in Samples 1 and 2 is approximately the same, but the naphthenes in Sample 2 is lower than in Sample 1. The lower content of naphthenes in Sample 2 could be explained by a higher conversion.27
A comparison of Samples 2 and 3 best illustrates the impact of catalyst type on gasoline hydrocarbon composition; they were produced from cracking hydrotreated HVGO on each catalyst type at identical operating conditions.
The zeolite in the maximum-octane catalyst is a USY-type and has a smaller unit cell size and, therefore, a lower site density than the CSSN zeolite used in the octane-barrel catalyst.
In catalytic cracking, the ratio of b-scission cracking to hydrogen transfer is higher when the zeolite has a lower acid site density.28 Gasoline, therefore, is produced with a higher olefins content with the maximum-octane catalyst as well as a higher percentage of the aliphatic hydrocarbons with a lower molecular weight.
Table 5 shows that in Sample 3 the percentage of aliphatic hydrocarbons (C5 + C6 + C7)/(C8 + C9 + C10 + C11 + C12) of 4.78% and the olefinicity of 42.4% are higher than those of Sample 2, which are 3.5% and 41.6% respectively.
The amount of aromatics and naphthenes in Sample 3 is lower than in Sample 2, which suggests that the maximum-octane catalyst has a lower cyclization activity.
Sample 3 contains fewer olefins than Sample 4. This is due to the higher catalyst:oil ratio and higher conversion despite the fact that Sample 3 was obtained at a higher reactor temperature.
The difference in aromatics content is marginal, but Sample 4 contains more naphthenes than Sample 3, due to a lower conversion for Sample 4.
Sample 6 resulted from cracking a virgin HVGO on the maximum-octane catalyst at an 8.5 catalyst-to-oil ratio and 511° C. reactor temperature. With these operating conditions, the FCC gasoline should contain the least amount of olefins of any sample. Sample 6, however, had one of the highest levels of olefins, 30%.
This sample also had the highest level of gasoline aromatics compared with other samples obtained from cracking the hydrotreated feedstocks on the maximum-octane catalyst. This confirms the conclusion that gasoline olefinicity is greater when cracking a virgin feedstock compared to cracking hydrotreated feedstocks.28
Fig. 4 shows the impact of FCC gasoline cut point on octane and olefins content.
A lower gasoline final boiling point is not desirable because octane decreases and gasoline olefins content actually increases.
Although increasing the reactor temperature and using a catalyst with a low equilibrium unit cell size (low hydrogen-transfer activity) increases FCC gasoline octane, gasoline olefins content also increases.
New methods are needed to produce high-octane FCC gasoline with low olefins content.
This could be achieved with a higher catalyst-to-oil ratio and with catalysts specifically designed to reduce FCC gasoline olefins while maintaining octane.29
New FCC reactor technologies may facilitate olefins reduction, since they give the opportunity to significantly increase the cat/oil ratio.30
Another option to minimize FCC gasoline olefins is a dual riser configuration in which the FCC gasoline is recycled to an additional upgrading riser and gasoline olefins are converted to LPG olefins (OGJ, Feb. 10, 2003, p. 52). F
References
1. Mott, R.W., Roberie, T., and Zhao, X., "Suppressing FCC Gasoline Olefinicity While Managing Light Olefins Production," presented to the 1998 NPRA Annual Meeting, paper AM-98-11, San Francisco, Mar. 15-17, 1998.
2. Upson, L.L., and Schnaith, M.W., "FCC Gasoline Desulfurization Options," presented to the 38th International Petrochemical Conference, Bratislava, Slovakia, Sept. 22-25, 1997.
3. Nocca, J.L., Cosyns, J., Debuisschert, and Didillon, B., "The Domino Interaction of Refinery Processes for Gasoline Quality Attainment," presented to the 2000 NPRA Annual Meeting, paper AM-00-61, San Antonio, Mar. 26-28, 2000.
4. Shorey, S.W., Lomas, D.A., and Keesom, W.H., "Use FCC Feed Pretreating Methods to Remove Sulfur," Hydrocarbon Processing, November 1999.
5. Miller, R.B., "Treating Option to Meet Clean Fuel Challenges," Petroleum Technology Quarterly, Spring 2001, pp. 69-74.
6. Lacijan, L.A., "FCC Refinery Solution for the European Market," Petroleum Technology Quarterly, Spring 2002, pp. 25-35.
7. "OATS For Ultra-low Sulfur Gasoline with Lower RVP and Octane Loss," IFP Industrial Division, Rueil Malmaison Cedex, France.
8. Martinez, N.P., Salazar, S.A., and Teiada, J., "Meet Gasoline Pool Sulfur And Octane Targets With the ISAL Process," presented to the 2000 NPRA Annual Meeting, San Antonio, Mar. 26-28, 2000.
9. Hydrocarbon Processing, "Refining Processes," November 2002.
10. Gentry J.C., "Novel Process for FCC Gasoline Desulfurization and benzene Reduction to Meet the Clean Fuel Requirements," presented to the 2000 NPRA Annual Meeting, paper AM-00-35, San Antonio, Mar. 26-28, 2000.
11. "Advances in Sulfur Technology," Hydrocarbon Engineering, Vol. 6 (2001), No. 9, pp. 45-49.
12. Balko, J., Wynn, N., and Bourdillon, G., "Novel S-Brane Technology fro Improved Ultra-Low Sulfur Gasoline Economics," presented to the European Refining Technology Conference, Paris, Nov. 18-20, 2002.
13. Krishnaiah, G., and Balko, J., "Reduce Ultra-Low Sulfur Gasoline Compliance Costs with Davison Clean Fuels Technologies," presented to the 2003 NPRA Annual Meeting, paper AM-03-121, San Antonio, Mar. 23-25, 2003.
14. Haas, A., Harding, D.A., and Nee, J.R.D., "New Catalyst Technology for Gasoline Sulphur Reduction," Catalagram, European Edition, Grace Davison, February 1999.
15. Harding, R.H., Vergopoulos, V., and Sobrinos, S., "FCC Catalyst Technologies for Gasoline Sulphur Reduction", Catalagram, European Edition, Grace Davison, February 2000.
16. Diarov, I.N., Batueva, I.U., Sadikov, A.N., and Colodova, N.L., Chemistry of Crude Oil, Chimia Publishers, St.Peterburg, Russia, 1990, p. 51.
17. Stratiev, D., and Minkov, D., "Prediction of FCC Yields from Feedstock Quality Characterised by Empirical Methods," Oil Gas European Magazine, Vol. 1 (2000), pp 27-32.
18. Cooper, B.H., and Donnis, B.B.L., "Aromatic Saturation of Distillates: An Overview," Appl. Catal. A, General 137, 1996, pp. 203-23.
19. Shiflett, W.K., et al., "FCC Feed Pretreatment to Control Sulfur in FCC Naphtha," presented to the 2002 NPRA Annual Meeting, paper AM-02-39, San Antonio, Mar. 17-19, 2002.
20. Harding, R.H., Gatte, R.R., Whitecavage, J.A., and Wormsbecher, R.F., "Reaction Kinetics of Gasoline Sulfur Compounds," presented to the 205th ACS National Meeting, Denver, Mar. 28-Apr. 2, 1993.
21. Cheng, W.-C., Kim, G., Peters, A.W., Zhao, X., and Rajagopalan, K., "Environmental Fluid Catalytic Cracking Tecnology," Catal. Rev.-Sci. Eng., Vol. 40 (1998), pp. 39-79.
22. Whitehurst, D.D, Isoda, T., and Mochida, I., Present state of the art and future challenges in the hydrodesulfurization of polyaromatic compounds, advances in catalysis, Academic Press, San Diego, Vol. 42 (1998), p. 345.
23. Diddams, P., Nee, J.R.D., and Paloumbis, S., "New Catalyst Systems based on Novel Zeolite and Selective Active Matrix Technology," Grace Davison FCC Technology Conference, Heidelberg, June 4-6, 1996.
24. Lakhanpal, B., "FCC Catalyst Technology for Short Contact Time Reactor Systems," Grace Davison FCC Technology Conference, Athens, Sept. 27-30, 1994.
25. American Petroleum Institute Research Project 45, 1957.
26. Marcilly , C., and Bourgogne, M., "FCC Gasoline: What is Behind Octane," presented to the ACS National Meeting, Sept. 10-15, 1989, Miami, p. 763.
27. Wallenstein, D., and Alkemade, U., "Modelling of Selectivity Data Obtained from Microactivity Testing of FCC Catalysts," Appl. Catal. A: General, Vol. 137 (1996), No. 37.
28. Haas, A., and Nee, J.R.D., "The Role of Zeolite and Matrix Activity in FCC Catalysts on the Molecular Weight Distribution of Vacuum Gas Oil Cracking Products," Erdoel Erdgas Kohle, July/August 1996, pp. 312-14.
29. Bourdillon, G., McQueen, D., and Nee, J.R.D., "New Catalyst Tecnology for Gasoline Olefins Reduction: GOAL Catalyst Series," Catalagram, European Edition, Grace Davison, February 2000.
30. Upson, L.L., Kauff, D.A., and Lacijan, L.A., "FCC Reactor Technology Developments," Grace Davison FCC Technology Conference, Heidelberg, June 4-6, 1996.
The authors
Georgy Andonov is deputy executive director of production for Lukoil Neftochim Bourgas, Bulgaria. During his 25 years with Lukoil Neftochim Bourgas he has served as an operator, shift supervisor, FCC process engineer, FCC unit manager, director of the refinery complex, and deputy senior engineer of production. Andonov holds an MS in inorganic chemistry engineering from Bourgas University.
Dicho Stratiev is deputy director of the research institute for oil refining and petrochemistry in the Lukoil Neftochim Bourgas petrochemical complex, Bulgaria. He joined Lukoil Neftochim Bourgas in 1990 as a process engineer in the catalysis laboratory in the research institute. During his 13 years with Lukoil Neftochim Bourgas, he has held positions as process engineer on various units. Stratiev holds an MS in organic chemistry engineering and a PhD in petroleum refining from the Bourgas University.
Dimitar Minkov is a professor of petroleum refining at Bourgas University, Bulgaria. His research interests include chemistry and technology of petroleum and gas, chemmotology, fuel additives, and environmental fuels. Minkov holds a PhD (1972) in chemical engineering in petroleum refining from the University of Chemical Technology and Metallurgy, Sofia, Bulgaria.
Slavi Ivanov is a professor of petroleum refining at Bourgas University, Bulgaria. His research interests include chemistry and technology of petroleum and gas, chemmotology, fuel oxidation and stabilization, fuel and lubricant additives, environmental fuels, and environmental equilibrium. He is chief editor of the international journals Oxidation Communications and Journal of the Balkan Tribological Association. He holds a PhD (1966) in chemical engineering in petroleum refining from the Bulgarian Academy of Science, Sofia, Bulgaria.