Suncor optimizes sulfur-recovery facility
Suncor Inc. Resources Group and Gas Technology Products LLC collaborated to significantly reduce plant operating costs while maintaining a high H2S removal efficiency in the LO-CAT unit located in Suncor's Spirit River, Alta., gas processing plant.
Suncor wanted the plant to remain emission free while running as economically as possible. This would guarantee the safety of plant operators and the environment.
Suncor Inc. Resources Group acquired the 7-22 Progress gas plant in 1996.
Due to the extremely competitive natural gas business, all gas processors, including Suncor, want to reduce operating costs. As part of Suncor's cost-reduction program, a close examination of the sulfur-recovery unit resulted in significant cost savings.
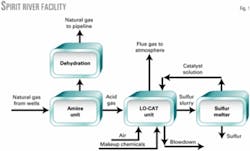
Suncor facility
The Suncor plant processes 20 MMcfd of natural gas at approximately 950 psig.
Fig. 1 shows that the processing train consists of an MDEA amine unit for removing CO2 and H2S, a glycol dehydrator and an autocirculation LO-CAT unit for removing the H2S in the amine acid gas prior to exhausting to the atmosphere.
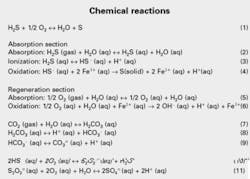
The proprietary LO-CAT process converts H2S to elemental sulfur using a multichelate iron catalyst. The overall reaction (See attached box, Reaction 1) is a modified Claus reaction; however, the mechanism for achieving this reaction is much different than what occurs in the Claus process.
The process is a liquid phase, ambient-temperature process; the Claus process, on the other hand, is a gas phase, elevated-temperature process.
The process includes an absorption and regeneration section. In the absorption section, H2S is absorbed from the gas stream into the circulating aqueous LO-CAT solution (Reaction 2). Once absorbed, H2S ionizes into hydrogen and hydrosulfide ions, and the hydrosulfide ions then react with ferric ions to form elemental, solid sulfur and ferrous ions (Reactions 3 and 4).
The ferrous ions cannot reduce any further; consequently, to have a continuous process they must be oxidized back to the ferric state. The process accomplishes this by sparging air through the effluent solution from the absorption section.
Oxygen is absorbed into the solution, which then oxidizes the ferrous ions back to the ferric state (Reactions 5 and 6).
Adding Reactions 2-6 yields Reaction 1 with all components in the gaseous phase except for sulfur, which is in the solid phase.
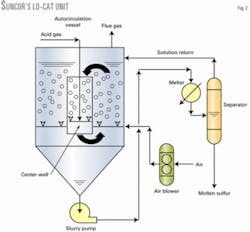
The LO-CAT unit at the Suncor plant was installed in 1990 and is an autocirculation design (Fig. 2). In this configuration, the absorption and regeneration sections are in the same vessel.
Acid gas from the amine unit is sparged into a center well (absorber), which is nothing more than an open-ended pipe located within a cone-bottom tank. Absorption reactions occur in the center well.
Regeneration air is sparged into the solution outside the center well where the regeneration reactions occur. Because there is considerably more regeneration air than acid gas, the solution outside of the center well is more aerated than the solution inside the center well.
This density difference creates a natural liquid circulation from the regeneration section to the absorption section. Thus, liquid circulation does not require pumps.
Sulfur settles out of solution into the cone section of the autocirculation vessel where it is removed as a 10-15 wt % slurry. The sulfur slurry is heated to approximately 120° C. with hot oil, and the solid sulfur is melted.
Molten sulfur and LO-CAT solution are separated, with the solution returning to the process. The melter system operates at approximately 3.5-barg pressure to prevent the aqueous solution from boiling. Molten sulfur solidifies and is then hauled to a landfill.
Due to the remote plant location and the relatively small amount of sulfur produced (2.2 tonne/day), it is more economical to dispose of the sulfur at the local landfill than to sell it to a remote user. Trucking the sulfur to a remote user costs more than what the sulfur is worth.
Even though the process produces water, a small amount of makeup is required to compensate for water consumed in saturating the regeneration air.
The plant originally used well water; however, plant personnel discovered that some of the problems they were experiencing in the unit, such as occasional foaming, disappeared when they switched to deionized water. The well water contained surface-active components that induced foaming. In general, LO-CAT units require potable-quality water.
Operators only need to test the solution for pH and reduction-oxidation (redox) potential once or twice a day.
Operating costs
There are two major sources of operating costs associated with a liquid redox process: chemicals and power consumption.
Major components of the Suncor's power costs were for two 65-kw rotary lobe blowers used for blowing air into the process. Major components of the chemical makeup costs are associated with iron and chelate chemicals and caustic addition to the unit.
Chelates are water-soluble, organic compounds that hold the iron in solution. The proper chelate will increase the solubility of iron in water from a few ppm to the 5 wt % range.
Chelates will, over time, oxidize via a free-radical mechanism and require replacement. A stabilizing agent, which acts as a free-radical scavenger, will allow the operator to control the chelate oxidation rate.
The LO-CAT process uses a proprietary method to reduce chelate degradation. A small portion of the H2S is converted to thiosulfate ions (S2O3=), which are excellent free-radical scavengers. Thiosulfate generation allows minimal chelate-replacement costs.
Iron is lost from the system when the solution is removed. For LO-CAT systems with sulfur melters, most iron is lost when during solution blowdown to control solution specific gravity.
Solution specific gravity depends on the amount of dissolved salts. Because LO-CAT operates at relatively low iron concentrations, chelated iron is not a major contributor to solution density.
In a LO-CAT unit, major contributors to solution density are carbonate and bicarbonate salts, and thiosulfate and sulfate salts.
Carbonate and bicarbonate salts form when gas streams containing CO2 are processed. Depending on the CO2 partial pressure and the desired solution pH, CO2 develops equilibrium concentrations of carbonate and bicarbonate (Reactions 7-9).
Equilibrium constants for each reaction increase with pH. Although the Suncor LO-CAT unit processes an acid gas stream with a high concentration of CO2, the system operates at atmospheric pressure, which yields a relatively low CO2 partial pressure. The carbonate and bicarbonate concentrations in solution are also low.
Thiosulfate formation is desirable as a method of controlling chelate degradation. If solutions become too oxidizing, however, thiosulfate can oxidize to sulfate, which has no beneficial value. The formation of thiosulfate and sulfate occur via Reactions 10 and 11.
Reactions 7-11 all produce acidic products. The operator must add caustic in some form (KOH, NaOH, or NH3), therefore, to maintain the solution in the slightly alkaline range, which is required to promote good absorption of H2S.
Thiosulfate and sulfate salts also contribute higher solution specific gravity.
If the dissolved salt concentrations increase unabated, one or both of two things will occur:
The solution will become less capable of absorbing oxygen and H2S.
The solution will eventually become saturated and salts will start precipitating from the solution resulting in plugging problems.
A small blowdown stream prevents this from happening. Systems that have a filter for sulfur removal produce a sulfur cake, which usually contains sufficient solution to compensate for salt formation. In a system that produces molten sulfur such as Suncor's, however, a liquid blowdown stream is usually required. These blowdown streams cause the process to lose iron, which requires makeup iron additions.
Unless the gas stream contains a hazardous or toxic component that is soluble in aqueous solutions, blowdown liquid will be only water containing dissolved salts; however, it will have a chemical and biological oxygen demand due to the chelates.
There is a cost associated with treating the blowdown stream. In Suncor's case, the blowdown is hauled away for remote treatment because there are no onsite wastewater treatment facilities.
Operating problems
The previous owner of the Spirit River facility paid little attention to the LO-CAT unit.
In general, overusing chemicals does not result in operating problems, just higher-than-required operating costs. In remote gas plants such as Spirit River, therefore, it is sometimes difficult to convince operators to change operating conditions if the unit is not presenting any operating problems. This is not Suncor's operating philosophy.
When it purchased the facility, Suncor contacted GTP and stated that the LO-CAT unit was not operating cost effectively. If chemical consumption, power consumption, and disposal costs of blowdown liquid were not reduced, the unit would be shut down.
The Suncor LO-CAT unit operates at close-to-design conditions of 123.4 cu m/hr of acid gas that contains 57.5 vol % H2S.
An initial examination of the unit and a review of historical operating data showed that the chemical addition rates were higher than the original design rates, and a higher-than-normal blowdown rate resulted in a lower-than-required solution specific gravity.
The iron concentration was maintained at more than twice the design level while the chelate concentration was more than three times the design level.
The system was designed to operate with one operating air blower; however, the unit was operating with both air blowers.
These conditions resulted in a positive solution redox potential, which promoted the formation of sulfate rather than thiosulfate resulting in a relatively high chelate-oxidation rate. This highly oxidized solution state resulted in high salt formation and a high blowdown rate to maintain the lower-than-design solution specific gravity.
The high blowdown rate resulted in a high iron-replacement rate and high solution-disposal costs. The unit was also experiencing symptoms such as occasional sparger plugging, which indicated that the solution was close to saturation.
Suncor optimization
The obvious remedy was to get the solution chemistry back to a normal state.
Suncor accomplished this by discontinuing the chemical addition of makeup chelate and makeup iron.
One air blower was turned off, which immediately cut power consumption by 50%. During this period, GTP monitored solution chemistry twice a week. In a relatively short time, the iron, chelate, and stabilizer concentrations, and the solution specific gravity reached design rates.
The chemical addition and blowdown rates were then reestablished but at rates corresponding to actual processing conditions. At the design conditions, power consumption, chemical costs, and disposal costs were lowered approximately 50%, thus achieving operating costs that were in line with Suncor's requirements.
Problems with sparger plugging were reduced. All of these reductions were achieved while maintaining an H2S removal efficiency of greater than 99.99+%.
Table 1 summarizes the operating costs.
Simply turning pumps and blowers off led to these cost savings. Suncor required no daily detailed chemical analyzes, just simple redox potential, pH, and specific-gravity readings.
Although it took years to get the LO-CAT solution in a state of near saturation, the situation was remedied in a matter of weeks.
The authors
Dean Freeman (DFreeman@ Suncor.com) is a production foreman at Suncor Inc. Resources Group, Calgary. He has 12 years' experience oil and gas industry, 6 of which have been with Suncor. Freeman has supervised several gas processing plants.
Jackie Barnette is in technical services at Gas Technology Products LLC, Schaumburg, Ill. She has 8 years of LO-CAT experience including technical support, research and development, analytical analysis support, and plant start-up. Barnette previously worked for ARI Environmental Inc. She holds a BA (1986) in chemistry and mathematics from Jamestown College, ND, and an MS (1988) in chemistry from Southern Illinois University, Edwardsville.
Gary J. Nagl is vice-president and general manager for Gas Technology Products LLC, Schaumburg, Ill., a wholly owned subsidiary of Merichem Chemicals & Refinery Services LLC, Houston. For the past 25 years, Nagl has been involved in the development and design of sulfur-recovery systems for different applications. Nagl holds a BS in chemical engineering from the University of Illinois. He is a member of AIChE, GPA, NPRA, and the Geothermal Research Council.