Refiners' Sulfur Dilemma: Phillips sulfur-removal process nears commercialization
Commercialization earlier this year of Phillips Petroleum Co.'s S Zorb sulfur-removal technology at the company's Borger, Tex., refinery represented a major step forward for the process.
The vertical start-up-from first feed to capacity production-was effected within 72 hr, and the high throughput of the unit at the refinery indicated that both the process engineering design and the unit's sorbent were efficacious. Start-up of the unit was uneventful and followed the expected timeline. Product gasoline with only 10-ppm sulfur was produced within 72 hr of unit start-up.
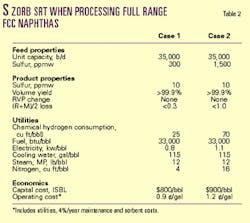
Within a week of start-up, the plant was processing 6,600 b/d of full-range FCC naphtha, 110% of the design capacity. The plant continues to produce low-sulfur, full-range FCC naphtha with minimal octane loss, meeting the gasoline sulfur-removal requirements for the refinery.
A group that included research and development, process operations, maintenance, safety, environmental, and construction personnel implemented the technology. Integration of teams from all these groups enabled the technology to move from concept to full operations in less than 18 months. Fluor Daniel Corp., Sugar Land, Tex., was responsible for process design, and Zachry Construction, Borger, Tex., constructed the unit.
Implementation of S Zorb technology started in 1998 in anticipation of the US Environmental Protection Agency's enactment of Tier II gasoline regulations that mandate refiners to produce an average gasoline pool with 30-ppm or less sulfur by 2006, with phase-in starting in 2004. Subsequently, the European Commission (EC) has stated that it expects the mandated sulfur level in gasoline and diesel to be lowered to 10 ppm in the near future.
The Z Sorb process can economically reduce the sulfur content of gasoline to less than 10 ppm with minimal octane loss, minimal hydrogen consumption, and near-zero volume loss. These results are obtained through the use of a novel sorbent that selectively removes sulfur from hydrocarbon molecules.
In conjunction with the sorbent development, a plant design was developed that allows continuous operation paralleling major refining units such as the fluidized catalytic cracker, thus reducing operation and maintenance costs.
Economics
Phillips wanted a sulfur-removal technology that would improve operating reliability and reduce operating and maintenance costs over the life of its unit. The cost of hydrogen, typically $2.50/Mcf, makes hydrogen consumption one of the most important considerations in the choice of sulfur-removal technologies. Consequently, the S Zorb process was designed for considerably lower net chemical hydrogen consumption when processing either diesel or gasoline.
In a 35,000-36,000 b/d unit, for example, the lower net chemical hydrogen consumption may decrease operating expense by up to $10 million/year, compared with hydrotreating, when producing ultralow-sulfur diesel (ULSD)1 and up to $2 million/year when producing ultralow-sulfur gasoline (ULSG).
In addition, the ULSG produced from the technology can maintain octane values 2-3 numbers higher than other methods when producing ULSG.1 This increased octane retention could yield an additional $5 million/year in benefits.
One result seen from the diesel pilot plant tests was the low chemical hydrogen consumption of the process.2 Based on the decreased hydrogen consumption alone, a diesel S Zorb unit could yield a net present value operating-expense savings of $16-32 million, assuming a weighted average cost of capital of 12%. The NPV calculation presented is based only on the first 5 years of operation, to be conservative.
In addition, ULSG generated by the S Zorb process has a higher octane number.1 The value of this octane retention is $5 million/year, based on a value of $0.20/octane barrel.3 Due to the decreased operating expenses and increased value of the low-sulfur gasoline, a gasoline S Zorb unit would yield 10-ppm sulfur gasoline with an NPV of $23.6 million for a 35,000-b/d unit.4
Gasoline
The S Zorb technology is not a modified hydrogenation technology; it uses a new sorbent that operates in a fluidized bed reactor.
In the fluidized bed reactor, a stream of vaporized gasoline and hydrogen is passed over the sorbent, yielding a low-sulfur product gasoline with little octane loss and no volume loss. The sorbent circulates between the reactor and a regeneration section allowing online regeneration and extended run lengths.
The run lengths of the S Zorb unit are designed to match the run lengths of typical FCC units. Matching the run lengths of the unit and the FCC unit allows refiners to remove sulfur from FCC naphtha when the FCC unit is in operation, decreasing costly refinery downtime.
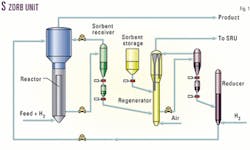
The Phillips process is in continuous operation, with the sorbent moving between three vessels in a semicontinuous process (Fig. 1). Sulfur is chemically removed from the gasoline stream in the reactor and stored on the sorbent.
The effluent stream from the reactor is a low-sulfur, full-range FCC naphtha with minimal octane loss, minimal hydrogen consumption, and little-to-no volume loss (Tables 1 and 2).
The sorbent is removed from the reactor in a continuous process and regenerated in an oxygen-containing atmosphere to produce sulfur dioxide, a small amount of carbon dioxide, and a fresh sorbent. The sulfur dioxide produced in this process can be separated from the regenerator effluent stream in a number of ways, depending on the refinery configuration.
The primary benefit of the absorptive process is the elimination of hydrogen sulfide from the reactor. The lack of hydrogen sulfide allows the S Zorb process to remove as much as 99+% of the sulfur from a gasoline stream without the need for an additional reactor to remove mercaptans formed from olefin recombination.
After oxidative regeneration of the sorbent, it is transferred to a small holding vessel where it is reduced by use of a hydrogen stream that can have as little as 50% purity. The sorbent reduction is highly invariant to reduction temperature and pressure. Therefore, the reduction vessel is primarily treated as a surge vessel and requires minimal oversight by the operators.
Key in the operation of the S Zorb unit is the ability to shut down the regeneration section of the unit for extended periods while the reactor continues to operate. This ability to isolate the regeneration section of the unit gives operators the flexibility to perform maintenance on the unit without interrupting the production of low-sulfur gasoline. In one instance at the Borger refinery, this flexibility allowed replacement of a compressor part without the entire unit being down.
Diesel
EPA has mandated that on-road diesel sulfur levels be reduced from the current level of 500 ppm to 15 ppm at the retail outlet by 2006. Following the S Zorb Sulfur Removal Technology gasoline pilot plant tests and concurrent with successful gasoline S Zorb Sulfur Removal Technology commercialization, Phillips demonstrated at the pilot scale that its process produces ultralow-sulfur diesel from currently available on-road blended diesels.
Tests conducted in pilot-scale reactors showed that the S Zorb process could remove sulfur down to less than 10 ppm from diesel with savings in operating expenses over hydrotreating. In fact, the S Zorb technology produced diesel with less than 1 ppm of sulfur under some conditions.2
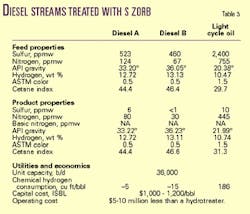
The process design for the diesel S Zorb unit is very similar to the design of the gasoline unit. It consists of a regenerator and reducer comparable to that of a gasoline S Zorb unit. The primary difference in the unit is an increase in pressure in the reactor (Table 3).
With these similarities in design and operation, a refiner can expect the same consistent operations in the diesel units as seen in the gasoline units.
In order to meet EPA's diesel requirement at the retail outlet, an effective sulfur-removal technology must be able to reduce sulfur levels in diesel to 5-8 ppm at the refinery gate. This low-sulfur level results from potential contamination of the diesel as it is transported to the retail outlet. S Zorb diesel sulfur-removal technology was able to meet those demands.
Two different feedstocks were desulfurized to less than 10-ppm sulfur in pilot-scale reactors. These two diesels came from refineries with significantly different configurations and crude slates. The diesel pilot-plant studies were carried out at temperatures of 700-800° F., 500-psig pressure, and a liquid hourly space velocity (LHSV) of 2.
The two feedstocks, labeled Diesel A and Diesel B (Table 3), represent typical finished highway diesels. They contain 20% and 17%, respectively, of hydrotreated light cycle oil (LCO).
At the same operating conditions, Diesel B was more amenable to production of ULSD than Diesel A. Otherwise the two finished diesels gave the same results, suggesting that most blended diesels are capable of being desulfurized to less than 10-ppm sulfur at these conditions.
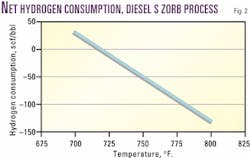
Net chemical hydrogen consumption is a strong function of temperature, and zero net chemical hydrogen consumption can be achieved under the same conditions used to produce ULSD (Fig. 2).
The dependence of hydrogen consumption on temperature gives a refiner the flexibility to adjust operating conditions to either run at zero net hydrogen consumption (minimizing operating expenses) or increase hydrogen consumption to increase the cetane index of the product ULSD (Table 3; LCO example).
If an increase in cetane index is not required, the refinery may operate at zero net hydrogen consumption. This low or zero hydrogen requirement may eliminate the need for a new source of hydrogen to meet the low-sulfur diesel requirements.
A decreased demand for hydrogen translates directly to lower operating expenses and to environmental benefits in reduced carbon dioxide and nitrogen oxide production. Thus, S Zorb sulfur-removal technology can provide refiners with flexibility.
The operating cost savings due to lower chemical hydrogen consumption is achieved with little or no changes to the product diesel after desulfurization. ASTM color is a specification for diesel in the US and is an indicator of the fuel's stability. The ULSD produced from Phillips' process had no observable change in ASTM color under typical S Zorb process conditions. Likewise, the diesel product had no significant change in ASTM color after 12 weeks of storage at room temperature.
Analysis of the ULSD produced from both Diesels A and B showed that there was no change in boiling range, API gravity, hydrogen concentration, or volume upon desulfurization.2
The sulfur-removal process leaves the hydrocarbon stream virtually unchanged except for the removal of the sulfur. Therefore, the product fuels are high quality fuels.
References
- "US Petroleum Refining: Assuring the Adequacy and Affordability of Cleaner Fuels," National Petroleum Council, 2000.
- Johnson, B.G., Kidd, D., Greenwood, G., and Slater, P.N., "Application of Phillips's S Zorb Process To Distillates-Meeting The Challenge," NPRA 2001 Annual Meeting, Mar. 18-20, 2001, New Orleans. La.
- Discussions with refiners.
- "S Zorb: Advances in Sulfur Technology," Hydrocarbon Engineering, September 2001.
The author
Jason Gislason is a senior chemist in the clean fuels group at Phillips Petroleum Co.'s research and development center. He is responsible for the development and commercialization of the S Zorb sorbent. Gislason graduated from Auburn University in March 1994 with a BS in aerospace engineering and earned a PhD in physical chemistry from South Dakota State University in December 1998. He currently is seeking a Masters of business administration at Oklahoma State University.