Long-term operating data shed light on Selectox process
Seven years' operating data from the world's largest Selectox unit have revealed valuable insights into how that process should work. Tom Brown Inc. operates the unit at its Lisbon gas plant in southeast Utah.
The Selectox process catalytically converts hydrogen sulfide (H2S) in dilute acid-gas streams to liquid sulfur without the need for a reaction furnace.
The Lisbon unit processes acid gas containing 4% H2S and 96% carbon dioxide (CO2), produces about 18 long ton/day of sulfur, and achieves 94% sulfur recovery at the start of a catalyst cycle.
Lisbon gas plant
The Lisbon plant is in Southeast Utah near the intersection of Arizona, Colorado, New Mexico, and Utah (Fig. 1). The plant is tucked into a remote red rock canyon about 30 miles east of Canyonlands National Park and 40 miles south of Moab, Utah.
Initial construction of the plant in 1960 by Pure Oil Co. consisted of inlet three-phase separation, oil stabilization, and compression. Sour gas, 60 MMscfd, was reinjected into the gas cap of the volatile oil reservoir. Oil production peaked at 11,000 b/d in 1966, and cumulative recovery is more than 50 million bbl of oil.
NGL recovery, full fractionation, and sour-liquids treating facilities were added in 1966. Liquid propane plus butane sales reached 280,000 gpd then declined slowly during the next 27 years. Produced gas continued to be recycled to the reservoir, supplemented by nitrogen injection for pressure maintenance.
Gas sweetening and conditioning facilities were installed in 1992-93, with gas cap blowdown and sale of residue gas commencing in September 1993.
The plant was operated by Union Oil Co. of California (Unocal) until July 1999 when Unocal's Rocky Mountain assets were acquired by Tom Brown Inc. (TMBR), Denver. TMBR is an independent exploration, production, and drilling company that focuses on developing principally gas resources in the Rocky Mountains of the US and Canada.
Sulfur unit
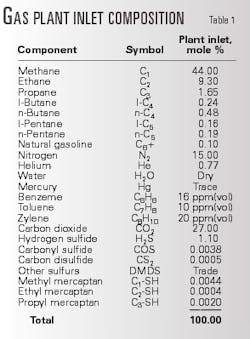
Inlet well stream gas production to the plant has averaged about 50 MMscfd of sour gas containing 27% CO2, 1.1% H2S, 15% nitrogen, 0.77% helium, plus hydrocarbons. Table 1 shows the inlet-gas composition.
The MDEA treating unit removes CO2 and H2S, yielding 12-16 MMscfd acid gas containing 4% H2S plus 96% CO2 and is the feed to the sulfur unit. Specialized on site gas-sample analysis has been used to identify trace compounds in the acid gas, including light hydrocarbons, aromatic hydrocarbons, and various sulfur compounds (Table 2).
The sulfur unit consists of the Selectox unit followed by a Beavon Sulfur Recovery (BSR) hydrogenation unit that conditions the feed to a tail-gas treating unit. Liquid sulfur produced in the Selectox unit is transported to Florida where it is used in the manufacture of fertilizer.
Understanding the Selectox unit requires examining certain operating events from the MDEA unit and also from the BSR unit.
Selectox reactor
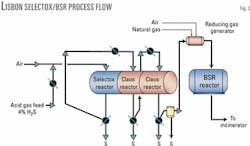
The Selectox unit consists of one Selectox reactor and two Claus reactors, plus associated equipment in series (Fig. 2). A fired reaction furnace is unnecessary. This unit is a "once through" process for acid-gas streams containing less than 5% H2S.
A recycle blower at the outlet of the Selectox reactor enables the unit to process acid gas with more than 5% H2S, but that has been unnecessary. The proprietary Selectox direct-oxidation catalyst was developed by Unocal and Ralph M. Parsons Co., but UOP now owns Unocal's rights to the technology.
The acid-gas feed flows through an inlet-gas sock filter, is preheated to about 400° F. (at 10 psig), then mixed with air before entering the reactor. The Selectox reactor is a refractory-lined pressure vessel loaded with catalyst supported by inert balls.
The exothermic reaction of H2S with oxygen to form sulfur and SO2 increases the acid gas temperature to about 700° F. within a few inches into the catalyst bed at start of run. The hot acid gas containing sulfur vapor is cooled to 300° F. in the condenser immediately beneath the reactor. The condensed liquid sulfur is drained from the bottom of the condenser into the sulfur-storage tank.
The Selectox catalyst has been replaced on three occasions since start-up, with the most recent changeout during August 2000 (current cycle).
Cycle No. 1 lasted 2 1/2 years, commencing September 1993 and ending February 1996. Cycle No. 2 lasted just 1 year, ending on March 1997. Cycle No. 3 lasted 31/2 years, ending August 2000.
The Claus reactors
Two Claus reactors are combined in a single refractory-lined pressure vessel separated by an internal partition. Each reactor is loaded with Claus catalyst supported by inert.
The acid gas leaving the Selectox condenser is preheated to about 400° F. (at 8 psig) using indirect heat exchange (heat medium oil) before entering the first Claus reactor. The exothermic Claus reaction of H2S with SO2 to form sulfur increases the acid gas temperature by 10-15° F. within a few inches into the catalyst bed, at start of run.
The hot acid gas containing sulfur vapor is cooled to 300° F. in the condenser immediately beneath the reactor. The condensed liquid sulfur is drained from the bottom of the condenser into the sulfur storage tank. The process is repeated for the second Claus reactor except the condenser following this reactor operates at a lower temperature (255° F.).
The tail gas leaving the second Claus condenser flows through a scrubber to remove entrained sulfur before entering the reducing-gas generator in the BSR unit at 6 psig. The Claus catalyst (both beds) has been replaced on four occasions since start-up, with the most recent changeout during August 2000.
The Claus catalyst has been replaced whenever the Selectox catalyst has been replaced; therefore, the dates of Claus Cycles A and B exactly matched Selectox Cycles 1 and 2.
The Claus catalyst was replaced less than halfway through the third Selectox cycle; therefore, Cycle C lasted 1 1/2 years ending July 1998 while Cycle D lasted 2 years until August 2000. The dates and duration of the Selectox and Claus catalyst cycles are summarized in Table 3.
Design basis
The process-design basis for the Selectox unit at Lisbon predicted sulfur-recovery efficiency to be 94% at start of run, declining with time to end of run with cycle lifespan expected to exceed 4 years. End of run occurs when the minimum acceptable efficiency is reached, which varies from 84 to 89% for Lisbon, depending upon inlet well stream gas rate and tail-gas unit performance.
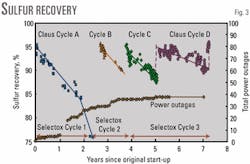
Fig. 3 shows the calculated temperature adjusted-recovery efficiency for the Selectox unit from start-up in September 1993 to August 2000. The dates of the three Selectox catalyst cycles are on the graph. However, four trends evident in this graph represent the four Claus catalyst cycles. The performance of the Claus catalyst beds must be considered to understand the operation of the entire unit.
Recovery efficiency for all new (Selectox and Claus) catalysts actually starts out at about 94%; however, cycle lifespan has been shorter than expected.
Selectox Cycle 1
The recovery efficiency of Selectox catalyst Cycle 1 started out at 94% then declined continuously until end of run in 900 days (2 1/2 years). There were several plant operating problems during this time responsible for the change in catalyst performance with time.
Power outages
The foremost problem at this time was more than 30 electric power outages; each caused an unexpected, rapid and complete shutdown of the entire plant. Sudden loss of power in the MDEA and Selectox units results in numerous undesirable and unstable operating problems.
Foaming of the MDEA solution and temperature excursions in the Selectox reactor are examples. The decline in catalyst recovery efficiency appears to be related to the cumulative number of power outages (Fig. 3).
The power failures were mostly due to utility company equipment and system operations. The problem was aggravated by such unexpected events as an airplane crashing into a power line, major regional power grid outages, forest fires, severe thunderstorms, and human error.
Plant and utility company personnel worked together to implement improvements in the power supply system, including installation of a new substation in June 1995.
The plant also completed internal improvements, including replacing the uninterruptible power supply (UPS) system and altering the manner in which faults cascade through the different motors in the plant. The result of this effort has been a significant reduction in power outages to the current 3-5/year.
Temperature excursions
Another cause of the decline in recovery efficiency in Selectox catalyst Cycle 1 was temperature excursions. The Selectox catalyst quickly catalyzes the various reactions between oxygen and other compounds present in the reactor, particularly H2S.
These reactions release heat; the more oxygen present, the more heat released. The ratio of acid gas to air should be between 8 and 10 scf/scf, depending upon several factors, including H2S concentration, air density, and target H2S to SO2 ratio at the back of the unit.
When the flow rate of acid-gas feed to the unit decreases sharply, then the volume of air that should be mixed with the feed must decrease accordingly or excess oxygen will be added. Temperatures in the Selectox catalyst exceeded the maximum recommended value of 750° F. on several occasions, which may have damaged the catalyst.
These temperature excursions were caused by flow disruptions in the MDEA unit, flow disruptions in the BSR unit, flow disruptions in the tail-gas unit, and by operator error.
Several improvements seem nearly to have eliminated the problem. A 2-in. ball valve for trim air flow control was placed in parallel with the primary air valve, which is an 8-in. butterfly valve. The large butterfly valve was unable to control air flow rate effectively during upsets.
Distributive control system changes allow better response to excess air flow situations, such as automatically closing the air valves when acid-gas flow dips or Selec tox reactor temperature exceeds 750° F.
The operators now tend to divert the acid-gas flow to the flare at a location upstream of the sulfur unit when an unstable situation develops.
It may be possible for oxygen to break through the Selectox catalyst bed and enter the first Claus catalyst bed during wide swings in flow rates. Free oxygen would severely damage the Claus catalyst, but we do not have solid evidence that this has taken place.
There have been several occasions when the temperature in the hydrogenation reactor increased by 50-100° F. following ratio swings. This rise is believed to be due to generation of excess SO2 in the Selectox bed and not due to oxygen breakthrough. The hydrogenation reactor converts SO2 to H2S exothermally.
Selectox Cycle 2
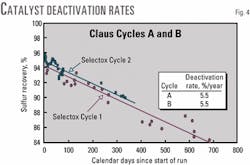
The recovery efficiency of Selectox catalyst Cycle 2 started out at 95% then declined rapidly until end of run in 1 year. The rate of decline in recovery efficiency of Cycle 2 was identical to that of Cycle 1, 5.5%/year (Fig. 4).
Feed stream contaminants
The electric power reliability problems had been solved by the start of Cycle 2, but contaminants in the acid gas feed to the Selectox unit, including heavy hydrocarbons, antifoam, and MDEA, had damaged the catalyst beds. Specific operating events in the MDEA unit are believed to be responsible for the change in catalyst performance with time.
MDEA unit
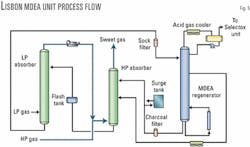
Fig. 5 shows the MDEA unit. Circulation is 1,500 gpm of 45 wt % generic MDEA to the high-pressure (HP) absorber operating at 700 psig.
Lean solution, 750 gpm, is fed to the top of the tower while semilean solution, 750 gpm, enters Tray No. 17 (30 trays total) in the middle of the tower.
This "split flow" configuration reduces energy consumption in the regenerator by using semilean solution that is not fully regenerated. CO2 content of the treated process gas out of the HP absorber averages less than 1,000 ppm(vol) with no measurable H2S. The "semirich" MDEA exiting the HP absorber (lean plus semilean) enters the top of the low-pressure (LP) absorber, which operates at 100 psig.
Hydrocarbons in the MDEA solution
Heavy hydrocarbons (ethane, propane, and heavier) had found their way into the MDEA solution via the inlet well stream gas because the gas filter located upstream of the HP MDEA absorber is a sock-type particle filter that inadequately removes hydrocarbon liquids from the gas.
Gas flow through the filter is from the outside annulus through the media to the inner hollow core of the element. The problem first became evident when we gathered several sets of MDEA samples from across the unit and had them analyzed by an independent laboratory for "oil and grease" or "total petroleum hydrocarbons" using EPA Test Method No. 418.1.
The results of these tests indicated that much of the oil and grease enters the solution in the HP MDEA absorber. The plant inlet gas flows through a three-phase separator then into the HP absorber. The three-phase separator cannot remove all of the heavy hydrocarbon droplets in the process gas.
To solve this problem, a true "gas/liquid" coalescer was installed downstream of the sock filter (and upstream of the HP absorber). The new coalescer dumps liquids to the drain about twice each day, which means it is working as intended, i.e., removing small droplets of liquid that passed through the sock filter.
Carbon filter
The activated carbon filter in the MDEA unit removes heavy hydrocarbons and other contaminants from the lean MDEA solution. The filter contains about 18,000 lb of activated carbon and filters a split stream of a third of the lean MDEA solution.
The carbon in the filter was replaced once during the first 6 years of operation (3 years of service per charge). During a turnaround in July 1998, 3 ft (95 bbl) of heavy hydrocarbon liquids were drained off the top of the lean MDEA surge tank. The surge tank is the logical quiet spot for such liquids to accumulate. The activated carbon is now replaced every summer.
Antifoam in the sulfur
The liquid sulfur in the rundowns from the Selectox condenser has turned red in color on several occasions. The red liquid sulfur would subsequently turn bright yellow upon solidification, and so it is still marketable.
Samples of the red sulfur were sent to an independent laboratory to identify the contaminant. Samples of antifoam, heat medium oil, an amine added to the boiler feed water, and MDEA were also sent to the lab.
The samples were analyzed by chromatography and compared. The antifoam appeared to be a fair-to-good match with the contaminant in the sulfur samples.
The heat medium oil, boiler feed-water amine, and MDEA were definitely not the contaminant. This result was repeated on one other occasion. It is hard to understand how antifoam can end up in the liquid sulfur because its boiling point and degradation temperature are greater than the bottoms temperature in the MDEA regenerator (250° F.).
Plant operating procedure for the first several years of operation was to add 2 gal of polyglycol antifoam to the MDEA solution, daily at first, but decreasing to once every few days. Additional antifoam was added during foaming incidents.
To reduce the volume of antifoam in the solution, the injection rate has been lowered to less than 1 quart/day. Continuous injection using a small chemical pump is still needed because the MDEA solution starts to foam if injection is stopped completely.
The carbon content of "bright yellow sulfur" is less than 200 ppm, while that of red sulfur ranges from 400 to more than 1,000 ppm.
The carbon in the sulfur may have come from the antifoam. However, it is also possible that the carbon in the sulfur came from compressor lubricants or heavy hydrocarbons from the inlet gas. Samples of compressor lubricants and heavy hydrocarbons were not compared with the contaminant in the red sulfur in the lab.
What is clear, however, is that contaminants in the acid-gas feed to the sulfur unit are contaminating the liquid sulfur produced in the reactors. The liquid sulfur has turned red on fewer occasions this last year than in the past.
We believe this is due to a reduction of hydrocarbons and antifoam entering the MDEA system.
Entrainment of MDEA
The acid gas feed to the Selectox unit is the cooled overhead vapor from the MDEA regenerator, and it will contain varying amounts of MDEA, depending upon operating conditions in the regenerator.
During normal operations, the concentration of MDEA in the liquid reflux is 1-2 wt %. In early 1995, it was discovered that the concentration of MDEA in the liquid reflux from the regenerator was more than 4 wt %. This seemed excessive, and the tower was entered in March 1997.
The top two trays had been completely destroyed by excessive hydraulic force from the inlet feed nozzle. The inlet nozzle fed the flashing two-phase rich MDEA solution directly onto the center of the top tray that caused failure of that tray and the one below it.
Loss of the top two trays, combined with an absence of wash trays above the feed nozzle (by design), resulted in MDEA entrainment in the vapor exiting the regenerator. Replacing the top two trays solved the problem; however, the inlet nozzle configuration was not changed. Predictably, the top tray was found to be buckled during the August 2000 turnaround and was again replaced. This time the inlet nozzle configuration was changed to eliminate the source of the problem.
Another solution to minimize MDEA entrainment in the acid gas was to convert the acid-gas cooler to a "giant evaporative cooler" (during the summer). The acid-gas cooler cools the overhead vapor from the regenerator in order to produce reflux and cool the acid-gas feed to the sulfur unit.
The air inlet ducts for this aerial cooler are covered with excelsior pads, and fresh water is sprayed across the top of the pads. This produces a sheet of water through which the air must pass. The water evaporates into the inlet air causing cooling. Results have been 10-20° F. cooling of the acid gas exiting the cooler, depending upon ambient humidity and temperature.
This additional cooling of the acid gas causes more condensation of liquids from the gas and, therefore, less entrained MDEA in the feed to the sulfur unit.
MDEA foaming
Foaming in the MDEA unit also causes entrainment of MDEA in the acid gas from the regenerator. The liquid sulfur turns very dark red immediately following severe foaming incidents. Laboratory analysis of dark red sulfur during Selectox Cycle 2 showed 60 ppm of nitrogen, which presumably comes from MDEA.
The more severe the foaming, the more liquid carries over into the scrubber at the start of the Selectox unit about 1,000 ft away from the regenerator. More liquid present in this scrubber implies that more liquid (containing some MDEA) also is carried into the reactors in the Selectox unit.
This is an on-going problem that we are working to solve by attacking the basic causes of foaming in the MDEA solution.
End of cycle
By March 1997, Selectox unit recovery efficiency had declined to an unacceptable 89%. Therefore, the catalyst was replaced in the Selectox and both Claus reactors.
However, stain tube testing indicated that the recovery efficiency of the Selectox reactor was about the same as it was at the start of the run, but recovery efficiencies of both Claus beds were much less than start of run. The Claus catalyst beds appeared to be seriously damaged. Nevertheless, all three beds were replaced with new catalyst.
Selectox Cycle 3
A problem developed during start-up of the unit immediately following replacement of all three catalyst beds. During the week-long shutdown of the plant, water condensed and accumulated in low spots in the 30-in. acid gas piping from the MDEA unit to the Selectox unit.
Subsequently during start-up, the acid gas flow was increased from 5 to 10 MMscfd, air was added to the acid gas, and the new Selectox catalyst started getting warm.
Water slug
The catalyst bed was rapidly brought up to operating temperature and pressure, and everything looked great when suddenly the temperatures in the catalyst bed plunged. In an instant, the reaction was quenched by a slug of water which moved into the reactor as the gas flow rate increased.
After a couple of hours the temperatures slowly started increasing and the reaction kicked off again. Unfortunately, the damage was done.
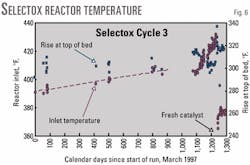
The entire 3 1/2 year run of Catalyst Cycle 3 was plagued by a bad spot in the upper center portion of the Selectox bed. The bad spot was characterized by sagging of the top center temperature indicator and sluggishness that actually led to a complete loss of reaction in the bed on two occasions.
The operators learned to control this problem by increasing the inlet acid gas temperature to the reactor. The higher inlet temperature prevented the sag and kept the reactor on-line. It was necessary to keep increasing the inlet temperature over the life of the catalyst cycle as shown in Fig. 6.
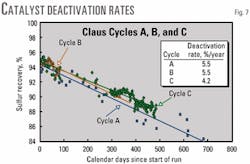
Claus Cycle C
The recovery efficiency of Selectox catalyst Cycle 3, Claus Cycle C, started out at 95% then declined rapidly for the first 500 days (Fig. 7). The rate of decline in recovery efficiency, 4.2%/year, was slightly less than that of the two previous cycles, 5.5%/year. During this time, few of the improvements in the MDEA unit, discussed above, had been implemented.
There was little improvement in the performance of the unit. The recovery efficiency of the Selectox unit declined to 88%, which was unacceptable, and the decision was made to replace catalyst beds.
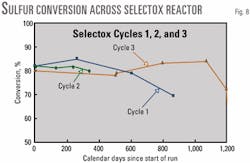
Stain tube testing across the individual reactors again showed that the recovery efficiency of the Selectox catalyst was unchanged from start of run, at 80% (Fig. 8). Recovery efficiency of both Claus beds combined was 8%, which was much less than that at start of run (15%). The Claus catalyst was seriously damaged. In July 1998, the catalyst in both Claus beds was replaced, but the Selectox catalyst was left in place.
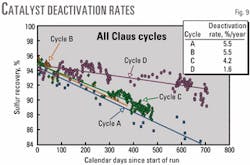
Claus Cycle D
The recovery efficiency of Selectox catalyst Cycle 3, Claus Cycle D, after the Claus beds were replaced with new catalyst, started out at 94% then declined during the next 700 days (Fig. 9).
This confirmed that the Selectox catalyst was essentially undamaged during Claus Cycle C. The rate of decline in recovery efficiency of Selectox Cycle 3, Claus Cycle D, was 1.6%/year, which was a huge improvement over the three previous cycles.
During this time, most of the improvements in the MDEA unit, discussed above, had been implemented.
The improvements in the MDEA unit apparently reduced the contaminants in the acid gas feed, which reduced the rate of damage to the catalyst beds.
There were no significant changes in Selectox unit operating practice during this time.
The authors
Steve Jones is a senior chemical engineer at Tom Brown Inc.' s Lisbon gas plant at Moab, Utah. He previously worked as an engineer for Mobil Oil Corp., Mesa Petroleum Corp., and Unocal. Jones was an engineer for Unocal at the Lisbon plant when it was acquired by Tom Brown. He is currently a project manager for the design and construction of a gas plant near Telluride, Colo. Jones received a BS in chemical engineering from the University of New Mexico in 1978.
Rick V. Bertram is a technical manager for UOP Hydro pro cessing Services, Anaheim, Calif. He is responsible for supporting UOP's tech nical services program for hydrocracking, hydrotreating, and sulfur recovery units. Before joining the company in 1995, he was employed by Unocal for 28 years in various engineering capacities. Bertram holds a Bachelor of Science degree in chemical engineering from the University of California at Berkeley.