HSE in Oil & Gas Operations Coalbed methane production faces numerous concerns
CBM ENVIRONMENTAL ISSUES-1
Operators contemplating developing coalbed methane gas (CBM) face numerous regulatory, residential, and legal concerns.
At stake are millions of dollars to assess, dispute, and settle claims regarding the impact of production practices.
This expenditure does not include the hidden costs for managing in crisis and redirecting a sparse labor pool to handle environmental issues.
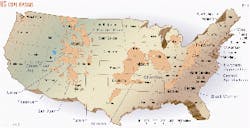
This first article of a two-part series summarizes allegations stemming from these concerns in the San Juan, Black Warrior, and Powder River basins (Fig. 1). The concluding part will discuss how operators can foresee and address in a more cost-effective way these concerns with a marginal amount of environmental base line information.
The most common complaints attributed to CBM practices in the San Juan, Black Warrior, and Powder River basins are as follows:
- Loss of domestic water quantity.
- Progressive deterioration of either surface or groundwater quality.
- Emergence of potentially dangerous concentrations of free and dissolved methane in both water and soils.
These complaints arise from a variety of causes, but this article will discuss information, contained in legal documents or published by regulatory agencies, that attribute these problems to CBM production practices.
Changes in water quantity, water quality, dissolved gas concentrations found in domestic water wells, or the perceived rate of methane seeps at the surface are naturally occurring phenomena, and one easily can attribute these phenomena to numerous factors other than CBM production operations.
CBM operations could not be assailed as easily if supported by data gathered in a base line sampling program conducted prior to and during the onset of CBM production.
Domestic water quantity
Several reasons can explain declining water yields in domestic water wells that might be completed in aquifers hundreds of feet above or below a producing coal horizon. A frequently quoted and widely held popular view is that drawing water from a coalbed aquifer is like drawing water from a glass through a straw. A lower water level in the glass will lower the water level in the straw.
This simplistic view is reinforced by the misconception that local aquifers have a regional extent and that they are both vertically and laterally homogeneous.
A more reasonable claim is that numerous permeable conduits can connect coal seams with overlying and underlying aquifers. These conduits include naturally occurring fractures, fractures induced by either underground mining or hydraulic fracturing, and poorly constructed producing or abandoned oil and gas wells.
The most pressing concerns, expressed by both state and federal regulators, are for CBM production in the proximity of basin margins. It has been asserted that downdip production can lower water levels in domestic water wells completed near or within outcropping CBM producing horizons.
Lowered water levels allegedly also may increase the risk for spontaneous coal combustion.
Surface, groundwater quality
Various sources cite that induced changes in the oxidation state of water in wells may be caused by changes in water quality attributed to CBM practices.
If one assumes that migrating coalbed methane can bubble through a domestic water well, the methane will displace free oxygen or generate conditions favorable for a chemically reducing environment. For example, bacterial consortia can assimilate the carbon in the organic methane molecule by forming simple building blocks for cell growth.1
Increased methane concentrations, therefore, can accelerate the activity of bacterial consortia living in water. This can have two effects.
The first effect increases the bacterial waste in water. These increased levels of bacterial cell matter and slime can turn clear water cloudy and cause it to appear unappetizing (Fig. 2).
The second effect lowers free and chemically bound oxygen levels that bacteria consume for respiration. A progressive loss of oxygen can promote the growth of iron-reducing bacteria (IRB) and sulfate-reducing bacteria (SRB).
SRB will strip sulfate ions of their oxygen during respiration. The byproduct of this metabolic process is carbon dioxide (CO2) and hydrogen sulfide (H2S) gas. The presence of H2S is unmistakable. It emanates as an offensive odor usually described as similar to the smell of rotten eggs. Water containing dissolved H2S is mildly acidic, and can corrode faucets, shower heads, and appliances.
IRB, on the other hand, will allow iron to readily dissolve in water. Dissolved iron and sulfide ions can combine to form suspended particles of iron sulfide that will impart a dark gray color to water. The iron quickly will oxidize and precipitate as an insoluble, rust-colored iron hydroxide when reduced waters rich in dissolved iron are in a home's plumbing system and come in abrupt contact with air. This precipitate can stain porcelain fixtures, faucets, and even discolor laundry (Fig. 2).
Bacteria can also be agents of chemical change in increasingly oxidized waters. If one assumes aquifer water levels fall as a result of CBM production, a water pump operating in progressively shallow water will draw more oxygenated water that is in closer contact with air.
If oxidation occurs in a wellbore containing dissolved iron, then iron-oxidizing bacteria will convert the iron to an iron hydroxide precipitate. This imparts a rusty red color to water and, if present in large concentrations, will render water opaque. The water will also tend to have an unpleasant taste, commonly described as metallic.
Various sources also cite that both chemical reduction and oxidation of domestic aquifers result from chemical reactions induced when produced water, discharged at the surface, infiltrates shallow aquifers.
Surface discharge also can affect surface water chemistry by changing salinity or modifying historic concentrations of metal ions.
Methane seeps
Methane seepage is the third and last type of complaint attributed to CBM production activities. Seeps pose the greatest potential safety hazard and engender the greatest fear among residents.
Methane is an odorless and colorless gas that can lead to spontaneous explosions if allowed to reach concentrations of between 5 and 15% by volume in air. This range is defined by the lower and upper explosive limit of methane, respectively. Such concentrations can be reached in two ways: by an exsolution of dissolved methane that is allowed to collect in an enclosed and unventilated space or by the accumulation of free gas seeping to the surface.
Methane seeps can displace normal oxygen levels in soil and kill vegetation, and extensive gas seeps can alter noticeably the vegetative landscape. The seeps also can behave as a carrier gas that transports undesired concentrations of H2S to the surface.
In sufficiently high concentrations, H2S irritates skin and eyes and, in the worst scenario, leads to loss of consciousness or death.
Litigators too easily explain the origin of dissolved methane in water wells and methane seeps as being from coalbed methane operations that lower the hydrostatic pressure and cause large quantities of gas to be released into the subsurface. Implied in this explanation is the idea that gas released from a coalbed methane reservoir has easy access to the surface and surrounding aquifers.
Both litigators and regulatory agencies have identified four migration mechanisms to account for methane in domestic aquifers and for methane seeps:
- Vertical migration through large, natural fractures that extend vertically from the producing reservoir to the surface or domestic aquifer.
- Gas migration along access paths provided by wellbore conduits. In the San Juan basin, for example, well installation practices conducted prior to the 1950s left the production casing annulus of deep oil and gas wells uncemented across both the shallower Fruitland formation and overlying strata.
Consequently, when CBM operations began in the 1980s, desorbed gas was free to migrate vertically from the Fruitland coal along the wellbore annulus and into shallow aquifer horizons.2
Domestic water wells can also provide gas conduits to the surface. For example, gravel packing of wells completed in the Wyodak coal seam was at one time an appropriate completion method for domestic water wells in the Powder River basin. Since CBM operations have begun, however, water yield in several such wells has declined, and the gravel pack has provided a conduit for gas to migrate to the surface. - Methane liberated during production could migrate updip until it emanates from the outcrop or shallow subcrop, near basin margins where gas seeps emerge along the outcrop belt of producing coal seams.
- Alternatively, down-basin production could lower water levels near the outcrop and allow gas to be released at the surface via in situ desorption of gas-saturated coal seams, also near basin margins where gas seeps emerge along the outcrop belt of producing coal seams.
Production-related complaints
It is essential that operators understand the natural plumbing dynamics of regional coalbed aquifers, and both overlying and underlying aquifers within 100 ft of a CBM producing horizon. Otherwise, their drilling, completion, and production methods may cause invasion water from outside the producing formation.
Under the right conditions, significant local cross flow between aquifers can occur both within and outside of the immediate wellbore environment. At best, this results in unnecessary costs for pumping out excess water. At worst, cross flow alters the geochemical properties of a CBM reservoir.
For example, cross flow can introduce dissolved sulfate into a reservoir causing sweet coalbed methane to turn increasingly sour. Operators can incur significant costs for monitoring, controlling, blending, and processing souring gas reserves.
Cross flow also can introduce fluids that promote wellbore scaling or corrosion. To control these effects, operators may have to inject expensive additives repeatedly into wells and reservoirs.
CBM operators continually evaluate their drilling and production strategies. Questions of greatest economic importance relate to issues such as optimum well spacing, and the impact of infill drilling on correlative rights.
Questions of this kind are usually left to the reservoir engineer to answer. Yet few operators realize that base line sampling of the producing reservoir and temporal monitoring techniques provide powerful and useful tools for both constraining and supporting reservoir engineering models.
Reference
- Erlich, H.L, Geomicrobiology, Marcell Decker Inc., 1996.
- Beckstrom, J.A., "Aquifer protection considerations of coalbed methane development in the San Juan Basin," SPE Formation Evaluation, 1993, pp. 71-80.
The author
Anthony W. Gorody is an environmental scientist and a principal with Universal Geoscience Consulting Inc., Houston. As an advisor and analyst for oil and gas companies, he has participated in defending major litigation involving coalbed methane operators in the US during the past 15 years. He also assists and trains managers to implement enviromental risk analysis when developing shallow gas plays. Gorody has an MS and a PhD in geology and geochemistry from Rice University, Houston.