Texas gas plant faces ongoing battle with oxygen contamination
Duke Energy Field Services (DEFS) is using oxygen (O2) scavenging and ion exchange to battle oxygen contamination at its Wilcox gas plant in Lavaca County, Texas.
Oxygen contamination in the amine system has resulted in degradation of the formulated MDEA (methyl diethanolamine) to bicine and heat stable amine salts (HSAS).
Plant operation
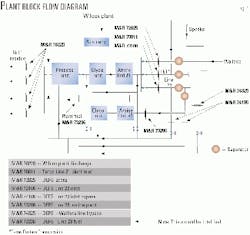
The Wilcox gas plant, commissioned by DEFS in spring 1999, was built to replace an existing lean-oil absorption plant that had been in service for 47 years. Capacity of the plant is 200 MMscfd of inlet gas received at 950 psig from four major gathering and transportation pipelines.
The inlet gas stream contains about 4% carbon dioxide (CO2), 2-3 ppm(vol) hydrogen sulfide (H2S), and 90-100 ppm(vol) of O2. The facility consists of inlet gas amine treating, inlet gas dehydration, inlet gas cooling, cryogenic liquids recovery, ethane fractionation, propane fractionation, and residue gas compression.
Residue gas enters a sales gas pipeline at 1,050 psig and a rate of 177.7 MMscfd. The liquid ethane product enters a pipeline at about 5,400 b/d at 1,320 psig. Approximately 2,500 b/d of propane and 6,500 b/d of butane and gasoline product are collected and trucked off site.
The cryogenic feed gas is treated to <50 ppm(vol) CO2 to meet maximum CO2 specifications in the recovered ethane product. Circulation rate of the inlet gas treater is 1,100 gpm with a 40 wt % solution of Ineos LLC's Gas/Spec CS-2000 amine.The residue gas is blended with bypassed inlet gas to meet a pipeline specification of 2% CO2. Any remaining inlet gas is treated through an additional 150 gpm side stream absorber that uses the same amine and regeneration system as the main absorber (Fig. 1).
Problems
Corrosion problems at the plant began about 30 days after start-up. The first problem was failure of the stainless steel (SS) internals of the six-stage, horizontal, high-pressure (HP) amine circulation pumps, which were lean-oil pumps retrofitted for amine service.
An investigation found 400 series SS corrosion. This was replaced with 300 series SS. Corrosion of 400 SS in certain amine service is well-documented, and the authors think this initial corrosion was a separate issue to problems discussed in this article.
After 4 months' operation, trouble was again detected in the amine-circulation pumps. Investigation of these pumps, as well as the rest of the plant, revealed major corrosion in the amine system, mainly to the carbon steel sections of the plant and most notably to the lean side of the plant.
The most significant corrosion was to the carbon-steel housing of the main amine circulation pump. This appeared to be caused by chemical attack. The body and impeller of the ductile iron amine booster pumps also showed significant corrosion in similar fashion to the main circulation pumps.
The level-control valve body, seat, and cage of the carbon-steel amine flash tank cage had to be replaced after a pin-hole leak was discovered. The carbon-steel seal-flush nipple on the main amine-circulation pumps failed and had to be replaced with 300 SS.
At the same time, corrosion was detected on the low-pressure suction nozzles of the carbon-steel main amine circulation pumps.
About 2 months after the main contactor plant start-up, the additional smaller contactor was placed in service. Two months later, the rich amine line from this additional contactor developed a pin-hole leak in the carbon steel valve body where the rich amine from both contactors combines and is fed to the regeneration system.
Root cause
From the beginning, all root causes pointed toward O2 contamination. Oxygen causes degradation of MDEA amines to form glycolate, acetate, formate, and oxalate.2 3 The DEFS Wilcox amine sample began to show consistent increasing levels of acetate, formate, and oxalate, in addition to thiosulfate.
Oxygen reacts with H2S to make thiosulfate in amine systems. The solution also showed other signs of O2 contamination through the degradation of MDEA to diethanolamine (DEA), monomethyl ethanolamine (MMEA), and triethanolamine (TEA). The degradation of tertiary amines like MDEA to secondary amines, such as DEA and MMEA, are also well documented.4
While O2 contamination was suspected as the probable cause of the corrosion, all other possible causes needed to be explored. Since corrosion was occurring in the main amine circulation pumps, the team investigated the mechanical design of the pumps to ensure this was not causing the corrosion.
Pump cavitation was the first potential problem investigated. While the pumps did not exhibit the characteristic cavitation sounds of "rocks in the pump," the net positive-suction head (NPSH) available was well above the vapor pressure of the amine.
It was decided to consider the possibility of erosion corrosion due to suspended particles in the amine. An amine sample was taken to a solids analysis lab for particle size tests. The lab concluded the particle size in the sample was in the 1-2 µm range and most likely did not contribute to the corrosion.
The next step was to analyze the inlet gas for O2. A third party analyzed the gas and determined the inlet gas had an O2 content of 90-100 ppm(vol). While 90-100 ppm(vol) O2 seems low, the Gas/Spec technology group (GSTG) has seen noticeable amine degradation and HSAS formation at these levels and below.
Table 1 shows the HSAS levels in the Wilcox amine system after corrosion was discovered. All the HSAS levels were below GSTG's maximum guidelines, which are based on maintaining carbon steel corrosion rates below 10 mil/year. The Wilcox plant was experiencing corrosion in its pumps to a much greater degree than would be expected with the level of HSAS in the system.
In addition to the HSAS and MDEA degradation products in solution, a rapidly increasing trend of soluble iron (Fe) in solution was noticed. Other plants operating with this same Gas/Spec amine, in similar applications, showed negligible Fe in solution.10 The level of Fe in the Wilcox system could not be accounted for by the level of HSAS in the system.
A DEFS engineer from another plant site, which experienced similar corrosion patterns, suggested the sample be analyzed for bicine.
Mass spectroscopy and gas chromatography found that bicine did exist in the amine sample, first showing levels at 600 ppm(wt). Previous research suggests that bicine levels in MDEA above 250 ppm(wt) will result in >10 mil/year corrosion of carbon steel.1 In addition, research of an MDEA plant that experienced severe lean side corrosion attributed that corrosion to bicine.5
Was the bicine causing the plant's severe corrosion? A review of the amine analytical data found that the bicine level closely trended the soluble Fe for the data (Fig. 2).
Research supports the trend in Fig. 2, noting that bicine has been shown to be potentially corrosive toward carbon steel.9 Since the plant was mainly experiencing corrosion in the carbon-steel sections, the root cause of the corrosion was determined to be bicine formation due to O2 degradation.
Bicine chemistry
Bicine [also called bis(2-hydroxyethyl)glycine] can form in MDEA-based amines when subjected to O2 contamination.1 4 There are several proposed mechanisms for bicine formation.
The first proposed pathway is the reaction of cyanide with formaldehyde to form bicine.5 Since the inlet gas at Wilcox is pipeline gas, neither of these compounds is likely to be present.
A second pathway involves the reaction of DEA with glyoxal (a known dialdehyde H2S scavenger) to form bicine.6 This mechanism can also be excluded as a possible pathway because the plant has adequate inlet gas filtration, and dialdehyde H2S scavengers are not known to be used in the pipeline that feeds the Wilcox plant.
A third possible pathway is the direct degradation of DEA with O2 to bicine. Recent data from several DEA plants with O2 contamination show significant bicine levels. Research does not show a direct degradation of DEA with O2 to bicine but it does indicate two moles of DEA can undergo a disproportionation reaction to form one mole of TEA and one mole of MEA.11
It has been reported that TEA further oxidizes with O2 to form bicine. Since the analysis does not show MEA present in the Wilcox system, the disproportionation of DEA to TEA and MEA can also be excluded as a possible pathway.
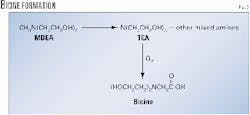
The fourth, and most likely, pathway to bicine involves a disproportionation reaction of MDEA to TEA and other mixed amines (such as DEA and MMEA) and further oxidation of TEA to bicine (Fig. 3).5
Bicine control strategy
With the root cause of the corrosion identified as bicine formation from the degradation of amine with O2, the next step was to determine how to control the bicine and O2.
The obvious first step was to try to eliminate the O2 source. The Wilcox plant is a straddle plant on a major pipeline with several hundred miles of upstream line, so identifying the O2 source would be nearly impossible. Further complicating this solution was the fact the pipeline O2 specification for this particular pipeline is less than 2,000 ppm(vol).
Since the plant was experiencing corrosion from bicine with only 90-100 ppm(vol) O2, the producers could still meet their contract O2 specification, and the bicine problem would not be eliminated. Therefore, eliminating the O2 source was not a solution, and other ways of controlling the O2 needed to be explored.
The next solution was to control the O2 and prevent it from further degrading the amine to form bicine. One possibility was to use O2 scavengers to possibly scavenge the O2 before it could form the bicine.
The first suggestion was to inject an O2 scavenger into the inlet gas to treat the gas before it entered the amine system. An alternative was to scavenge the O2 that was absorbed into the amine.
Scavenging the O2 out of the gas, however, would require much higher chemical usage than scavenging out of the amine. That is because the solubility of O2 in amine for this plant is calculated to be 2-10 ppm(vol) based on research data.8 The amount of scavenger needed to remove 2-10 ppm(vol) should theoretically be much lower than the amount needed to remove 90-100 ppm(vol) in the inlet gas.
Several possible O2 scavengers were reviewed, and a commercial amine corrosion inhibitor was tested which also acts as an O2 scavenger. The objective was to react the O2 with the scavenger before the O2 could further degrade the MDEA to secondary amines and bicine.
Although this scavenger would not remove the bicine or secondary amines already in the system, the plant hoped it would prevent further degradation. As Fig. 4 shows, the scavenger greatly reduced the bicine buildup rate from 60 ppm(wt)/day (21.1 lb/day) in September 1999 to 6 ppm(wt)/day (2.1 lb/day) in October-December 1999 until the solvent was reclaimed via ion exchange.
After completing partial ion-exchange reclamation (discussed later), there appeared to be a spike in bicine level. During this same time period, a miscommunication caused a shutoff of the inhibitor flow to the plant. This spike in bicine was caused by the lack of inhibitor flow.
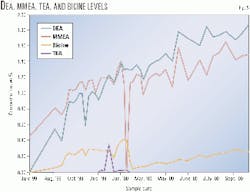
While the inhibitor-scavenger was effective in reducing the bicine buildup rate, it was not effective in slowing or stopping the formation of secondary amines, such as DEA and MMEA (Fig. 5). The TEA level remained very low in the system, but it is believed this is because the reaction of TEA with O2 to form bicine (Fig. 3) is quicker than the rate of formation of TEA from MDEA degradation with O2. Therefore, the TEA will not remain in the system but be converted to bicine.
Before the start of O2 scavenger addition, caustic soda (NaOH) was used to neutralize the bicine in the amine. The objective was to neutralize the bicine by converting it from an amine-bicine molecule to an Na-bicine molecule and reduce the corrosion due to the bicine already in the system.
Caustic neutralization has been shown to reduce the corrosivity of bicine in lab studies.1 The amount of NaOH needed to neutralize 80% of all the anions in the amine was calculated to prevent over-neutralization. These anions included bicine, thiosulfate, formate, oxalate, and acetate.
After adding the calculated amount of NaOH, analysis determined the bicine and HSAS had been 70% neutralized. The results of this neutralization are rather uncertain, because the plant did not have objective data measurement of corrosion rate (i.e., corrosion coupons or IR probes) before and after the neutralization.
Subsequently, the plant did not see any reduction in corrosion attack on the circulation pumps.
After addition of NaOH to neutralize the bicine and the addition of an O2 scavenger, the plant partially achieved its goal of minimizing further bicine formation by reducing the buildup rate.
However, corrosion was still found in the main amine circulation pumps. The corrosion continued to manifest itself in reduced output of the pumps due to erosion of the carbon-steel pump housing.
More ways were needed to further control the corrosion, not by just minimizing the formation of bicine, but also by removing the bicine from the system.
Options
The first idea was to continuously purge amine from the system to establish an amine loss rate that matched or exceeded the bicine buildup rate.
The amine loss rate at the Wilcox plant was below 0.25-0.50 lb/MMscf, which is known to be the industry average for amine loss rate in high-pressure natural gas plants. That would probably be effective in reducing and controlling the buildup rate, but it would not solve the problem.
The next option considered was ion-exchange reclamation. Ion exchange is effective in removing HSAS as well as bicine from amine plant solutions.9 An ion-exchange bed and piping were installed to manually reclaim a slip stream of the amine. The bed size installed consisted of 75 cu ft of strong-base anionic resin.
The plan was to treat the amine at a flowrate of 48 gpm for 4 hr and then regenerate the resin with NaOH for 12 hr. The plant followed this procedure, completing one cycle a day for 3 weeks with periodic samples of the amine system analyzed to determine if the bicine level was dropping.
After 3 weeks of manual reclamation, analyses showed the resin was somewhat effective in removing anions but not the bicine. Since research shows that bicine can be removed via ion exchange, it was still believed bicine removal was feasible.
After completing ion exchange calculations, it was determined that the resin bed installed was too small to overcome the bicine buildup rate and that a much larger resin bed would be needed. A third-party reclamation vendor, it was concluded, would be better able to reclaim the solvent using ion exchange.
Since NaOH was added to neutralize the bicine, the bicine and HSAS could not be completely removed from the system due to the Na cations (Na+)in solution.
It is well known in industry that when Na+ is present in an amine system, the Na+ must be removed from the system before the contaminant anions can be removed. Therefore, after partial reclamation, the third party had to install a cationic resin bed ahead of the anionic resin bed.
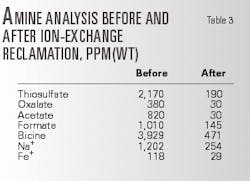
The amine was treated in the cationic bed to remove the Na+ and then treated to remove the HSAS and bicine anions with the anionic bed. Table 2 shows the effectiveness ion exchange had in reducing the plant contaminant levels with the manual reclamation as well as after partial anionic exchange reclamation by the third party.
The analytical data from ion-exchange reclamation produced some interesting facts. Ion exchange was effective in removing the HSAS and bicine, but the anions appeared to exhibit different affinities for the resin. Thiosulfate showed the strongest affinity, while bicine had the weakest affinity.
This behavior of varying resin affinities for thiosulfate, oxalate, acetate, and formate is supported by previous published research but not the relative affinity of bicine for resin.12
The analytical data confirmed bicine was removable with ion exchange, but it had the weakest affinity for the resin and would be the most difficult anion to remove from the Wilcox system.
Table 3 shows the results of complete ion-exchange reclamation by the third party. The data confirm that ion-exchange reclamation would remove bicine from the amine system effectively. However, plant engineers were not comfortable that the bicine level would remain low, because the O2 still existed in the inlet gas, and the O2 scavenger reduced but did not stop the bicine buildup rate.
The plant needed some way to remove bicine continually from the system as it accumulated. In the meantime, corrosion inhibitor-O2 scavenger minimized the buildup while engineers looked at the possibility of installing a permanent ion-exchange unit.
Permanent ion-exchange units in amine plants are becoming more common, particularly in refinery applications where HSAS levels are high. However, installation of a permanent unit in a gas plant to remove bicine is not common. As discussed earlier, the ion exchange results revealed the anions showed varying affinities for the anionic resin.
Since bicine removal was the main objective, GSTG and the Wilcox personnel worked with the supplier of a permanent ion exchange unit to ensure removal would be obtainable.
To see if the unit would work for this particular application, a 55-gal drum sample of the amine was sent to the ion-exchange unit manufacturer for testing. The sample was fed to the pilot plant ion-exchange unit to predict the Wilcox plant expected performance.
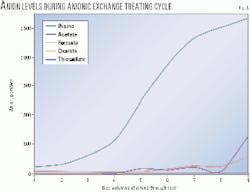
The treated amine was sampled after each volume of amine (equivalent to the resin bed volume) was passed over the resin.
Fig. 6 shows the anion levels of the treated amine stream with each bed volume of amine. As can be seen, the treated bicine level increases with each bed volume to a much greater extent than the HSAS. Therefore, to remove the bicine effectively, the ion-exchange treating cycle had to be shortened over the typical cycle time required to remove only HSAS.
Also, the resin would require more-frequent regeneration. That means a much larger ion-exchange unit than would be needed to remove the same level of anions, assuming only HSAS.
Using the above data, and assuming a bicine buildup rate of 6 ppm(wt)/day (2.1 lb/day), the unit was built to preferentially remove bicine. This unit was installed at the plant in August 2000 and is currently being implemented.
While the unit worked well in a pilot plant setting, the plant continues to work out operational bugs in the unit and has not as yet seen a reducing trend of bicine.
In addition to these operational bugs, the buildup rate of bicine since the third-party ion-exchange reclamation has shown to be 50% higher at 9 ppm(wt)/day (3.2 lb/day). Therefore, the actual performance of the unit may not reflect the design performance due this change in plant condition.
Results
While the root cause of the plant's problems was quickly identified, controlling the bicine formation and stopping the corrosion has been a difficult task.
Oxygen scavenging the amine has greatly reduced, but not stopped, the bicine formation. Ion exchange removed the bicine, but with more difficulty than expected due to its relatively weak affinity for the resin.
Ion-exchange reclamation of the amine greatly reduced the corrosivity of the solution, particularly to the carbon-steel housing of the main circulation pump. However, buildup of the bicine recurred during implementation of the permanent ion-exchange unit, and carbon-steel corrosion returned.
References
- Rooney, P.C., Bacon, T.R., and DuPart, M.S., "Effect of Heat Stable Salts on Solution Corrosivity of MDEA-based Alkanolamine Plants, Part III," 47th Annual Gas Conditioning Conference, Norman, Okla., 1997.
- McCullough, J.G., and Nielsen, R.B., "Contamination and Purification of Alkaline Gas Treating Solutions," Corrosion/96, NACE, Houston, Paper No. 396, 1996.
- Rooney, P.C., Bacon, T.R., and DuPart, M.S., "The Role of Oxygen in the Degradation of MEA, DGA, DEA, and MDEA," 48th Annual Gas Conditioning Conference, Norman, Okla., 1998.
- Critchfield, J.E., and Jenkins, J.L., "Evidence of MDEA Degradation in tail gas treating plants," Petroleum Technology Quarterly, Spring 1999, pp. 87-95.
- Green, J.G., "Identification of Corrosive Agent in an Industrial Process: Mass Spectrometry as a Part of a Multi-technique Problem Solving Effort," 39th ASMS Conference on Mass Spectrometry and Allied Topics, May 1991.
- Chakma, A., and Meisen, A., "Identification of Methyl Diethanolamine Degradation by Gas Chromatography and Gas Chromatography-Mass Spectrometry," J. Chromatography, 1988, 457, 287. b) Chakma, A., and Meisen, A., "MDEA Degradation with CO2," 35th Canadian Chemical Engineering Conference, October 1985.
- Farfan, N., Cullar, L., Aceves, J.M., and Contreras, R., "High-yield Synthesis of N-(2-Hydroxyethyl)-N-alkylgylcine Derivatives by Reaction of Ethanolamines with Glyoxal," Synthesis, 1987, 10, 987.
- Rooney, P.C., and Daniels, D.D., "Oxygen Solubility in Various Alkanolamine/Water Mixtures," Petroleum Technology Quarterly, 1998, 3(1), 97.
- Cummings, A.L., Veatch, F.C., and Keller, A.E., "Corrosion and Corrosion Control Methods in Amine Systems Containing Hydrogen Sulphide," Materials Performance, January 1998.
- Sargent, A.L., Rooney, P.C., Stewart, E.J., Van Landingham, J.L., and Seagraves, J.P., "New Deep CO2 Removal Solvent Useful for Natural Gas, Coal Seam Gas, and Refinery Applications," 50th Annual Gas Conditioning Conference, Norman, Okla., 2000.
- Private discussions with Peter Rooney, Dow Chemical Co., 2000.
- Keller, A.E., Kammiller, R.M., Veatch, F.C., Cummings, A.L., and Thompsen, J.C., "Heat-Stable Salt Removal from Amines by The HSSX Process using Ion Exchange," 42nd Annual Gas Conditioning Conference Norman, Okla., 1992.
The authors
Mike Howard is an asset engineer with Duke Energy Field Services in Warda, Tex. For the past 6 years, he has provided technical support for many of the company's natural gas processing plants in Texas and Louisiana. His experience is in the design, computer modeling, project management, troubleshooting, and start-up of various natural gas processes including amine treating, glycol and molecular sieve dehydration, cryogenic plants, pipeline gathering systems, compression, and CO2 reinjection. Howard holds a BS in chemical engineering from Texas A&M University, Kingsville.
Andy Sargent is a technical services engineer with Ineos Gas/Spec technology group in Freeport, Tex. For the past 5 years, he has been involved in formulated-MDEA technical support in natural and refinery gas and liquid amine processing. Sargent's experience includes amine plant computer modeling, design, troubleshooting, and optimization. He holds a BS in chemical engineering from Texas Tech University, Lubbock.
Presented at the 51st Laurance Reid Gas Conditioning Conference, University of Oklahoma, Norman, Feb. 25-28, 2001.