GTL: Progress and Prospects - Development progresses for GTL fuels, specialty products
Newer gas-to-liquids (GTL) technologies are being developed to achieve better economics at smaller plant sizes. GTL technology supplier Syntroleum has now developed its technology to the stage that a commercial plant can be built.
The first commercial-scale application of the Syntroleum GTL technology will be the Sweetwater plant, a specialty chemicals and lube oil plant on the Burrup Peninsula in northwestern Australia. In November 1999, Tessag Industrie-Anlagen GmbH began engineering for this 10,000-b/d grassroots plant.
Fischer-Tropsch development
In the first part of the 20th Century, the world was moving from a coal-based economy to a petroleum-based one. This trend concerned developed nations, which had abundant coal, but lacked sufficient oil to achieve their economic and political goals.
In Germany, a concerted effort was begun to convert coal and petroleum coke into liquid hydrocarbons. In 1923, Franz Fischer and Hans Tropsch discovered the process that bears their names and that had potential to meet these objectives.
This process was first commercialized in Germany in 1936, followed by plants in France and Japan during World War II and then South Africa after the war.
Other than in South Africa, interest in the Fischer-Tropsch process waned as significant oil reserves were discovered, and interest in nuclear power increased.
The energy crisis of the 1970s and concerns about problems with nuclear power caused a brief resurgence in interest. But sustained interest in the process has largely been associated with the desire to convert stranded natural gas into liquid hydrocarbons and with concerns over the environmental impact of sulfur and aromatics in fuels derived from petroleum.
The first step in the GTL technology is the production of synthesis gas (H2 and CO) from natural gas.
In a second step, synthesis gas ("syngas") is converted on a Fischer-Tropsch catalyst to liquid hydrocarbons ("Fischer-Tropsch liquids"). The liquids contain no detectable sulfur and can be converted by mild hydrotreating into highly saturated synthetic fuel.
Tests with synthetic fuel have shown that the emission of environmental pollutants was lowered notably relative to conventional diesel fuel.
Another utilization of Fischer-Tropsch liquids is the production of lubricants and specialty chemicals. The synthesis is essentially the same as for the production of synthetic fuel. The main difference lies in the further processing of the liquids. Lubricant production requires hydro-isomerization of normal paraffins.
Syntroleum's GTL technology
Syntroleum's GTL process has been under development since the early 1980s. The process utilizes the same principal steps as other Fischer-Tropsch processes: production of syngas from natural gas and conversion of syngas into Fischer-Tropsch liquids on a catalyst.
The goal of Syntroleum's development was to be commercially viable at plant capacities that would allow the use of a significant portion of the world's remote gas reserves.
To achieve this goal, proprietary cobalt-based Fischer-Tropsch catalysts with high activity and high selectivity were developed and the complexity of each processing step was simplified. This led to several significant differences in the execution of the process steps compared to other Fischer-Tropsch technologies.
Syngas is generally produced from natural gas with either gas-phase partial oxidation (POX) or steam-methane reforming (SMR).
In the POX process, natural gas reacts with pure oxygen in an open flame at temperatures of 1,200-1,500 degrees C. The gas phase POX produces syngas with an H2-to-CO ratio (H2/CO) of typically less than 2:1, on a molar basis.
The SMR process converts natural gas with steam on a nickel catalyst at 800-1,000 degrees C. to a hydrogen rich syngas. The typical H2/CO ratio is greater than 3:1 (without CO2 recycle). The natural gas must be desulfurized to prevent catalyst deactivation.
A third commercially applied process-autothermal reforming (ATR)-combines POX and SMR in a single step. The benefits are a lower reaction temperature, lower oxygen consumption, and a H2/CO ratio of 2:1 that is ideally suited for the Fischer-Tropsch synthesis.
An expensive cryogenic air-separation plant is required, however, for oxygen production in both POX and ATR processes.
The Syntroleum process utilizes a proprietary ATR reactor catalytically to convert natural gas to syngas using air instead of pure oxygen to avoid the cost and safety issues of an air-separation plant.
Because the system is in heat balance, no heat-transfer system is required, and the ATR reactor is much more compact than is possible with SMR or POX. The H2/CO ratio of the resulting nitrogen diluted syngas is close to 2:1 and can be adjusted up or down by altering the amount of steam added to the reaction.
The main reactions occurring in the ATR reactor are defined for methane (CH4) in Equations 1-3 (see accompanying equations box). For higher hydrocarbons similar reactions occur.
The syngas at the reactor outlet is in equilibrium with respect to the reactions and is soot-free.
The ATR reactor is a refractory-lined pressure vessel containing a catalyst system specifically developed for the ATR in conjunction with a leading manufacturer of syngas preparation catalysts. The proprietary design allows steam-to-carbon ratios (S/C) less than those typically used in conventional ATRs (1.7:1 to 2.2:1).
Lower S/C ratios are favorable because this significantly improves the economics of an ATR-based Fischer-Tropsch plant.1
By selection of the correct S/C ratio, oxygen-to-carbon ratio (O2/C), and operating temperature, the syngas composition can be optimized for the downstream Fischer-Tropsch synthesis.
The viability of the proprietary Syntroleum ATR design was successfully demonstrated in two pilot plants for a broad range of process conditions.
The pilot plant within a refinery at Cherry Point, Wash., demonstrated more than 6,000 hr of operation.
The Cherry Point ATR was sized to generate sufficient syngas for the downstream 70-b/d Fischer-Tropsch pilot unit. The feed to this ATR for most experiments was natural gas from a pipeline. For some experiments, additional gases were added from trailers to simulate alternate feed compositions.
The ATR at Syntroleum's pilot plant in Tulsa is designed to provide syngas for a 2-b/d Fischer-Tropsch pilot unit that has been in operation since 1991.
Fischer-Tropsch synthesis
The Fischer-Tropsch synthesis converts syngas to hydrocarbons of different chain lengths. Over the years, various catalyst and reactor systems have been developed and tested in academia and industry.
Generally, the reactions take place under moderate temperatures (200-300 degrees C.) and moderate pressures (10-40 bar; 145-580 psi) utilizing iron or cobalt-based catalysts. The conversion-per-pass is generally low, thus necessitating gas recycle to increase overall product yield.
The chain length of the Fischer-Tropsch hydrocarbons depends on such factors as temperature, type of catalyst and type of reactor employed. The chain length distribution follows an Anderson-Schultz-Flory distribution function characterized by the chain-growth probability factor a.
An increase in the a value equals a higher wax selectivity. The practically achievable a value is in the range of 0.90-0.94, with cobalt catalysts tending to achieve higher a than iron.2 The Fischer-Tropsch reaction is defined in Equation 4 in which "-CH2-" represents a product consisting mainly of linear paraffinic hydrocarbons of variable chain length.
Higher a is desirable because it increases the yield of the most valuable products when refined. In the case of a fuels plant, the yield of middle distillates can be as high as 80% with a high a catalyst.
Depending on the reaction conditions and the type of catalyst, additional products are obtained. Especially with iron catalysts, substantial olefins, alcohols, and sometimes aromatics can be produced. Ketones and acids are obtained in minor concentrations. With cobalt-based catalysts, only small amounts of olefins and alcohols and no aromatics are produced.
In general, Fischer-Tropsch liquids are sulfur free because sulfur is removed from the natural gas feedstock.
Syntroleum developed proprietary cobalt-on-alumina based catalysts. These are highly active and produce a paraffinic hydrocarbon mixture with a high a value and minor amounts of mono-olefins and primary alcohols.
High conversion can be achieved in a single pass, eliminating the need of gas recycle. This is a fundamental requirement when utilizing syngas production with air because the generated syngas contains almost 50% nitrogen.
The nitrogen passes through the system and leaves the Fischer-Tropsch process together with unconverted syngas and minor amounts of gaseous hydrocarbons as tail gas. This low-btu tail gas (low because of nitrogen dilution) is used as an energy source to increase the overall efficiency. It can either directly be utilized in gas turbines or as a heating medium in the process.
The catalysts have been tailored for two different types of reactor systems, a conventional multitubular fixed-bed reactor and a moving-bed reactor.
Multi-tubular fixed bed reactors are proven technology in, for example, methanol synthesis. They have low scale-up risks because the scale-up from one tube to several thousand parallel tubes is a straightforward process.
Moving-bed reactors are simpler in design, less expensive, and allow a continuous catalyst regeneration outside the reactor but have a higher scale-up risk, which can be mitigated by large-scale pilot plant experience.
These pilot plant tests were carried out to demonstrate Syntroleum Fischer-Tropsch catalyst performance and to gain operating data over a broad range of process conditions. A moving-bed Fischer-Tropsch reactor was successfully operated at the Cherry Point, Wash., refinery for more than 6,000 hr.
In this reactor type, catalyst particles are suspended by gas in circulating hydrocarbon products. This type of reactor is very efficient because the multiphase mixing leads to excellent heat removal resulting in minimized heat-transfer area. Life tests showed that the catalyst possesses very good stability with respect to activity and selectivity.
The catalyst attrition resistance is excellent and has been monitored using particle size analyses and particle settling characteristics. Furthermore, the continuous separation of the catalyst from the liquid products was demonstrated successfully.
Tests with tubular fixed-bed reactors were performed at Syntroleum's Tulsa pilot plant. The capacity of the reactors is 2 b/d of liquid products. The pilot plant reactors were equipped with tubes of commercial diameter and length.
Since the pilot plant began operation in 1991, thousands of hours of operating experience have been logged. The pilot plant activities will be continued for further technology development and optimization.
Synthetic fuels
In recent years, diesel-engine development has made advances in meeting greater fuel efficiency and lower emission rates of environmental pollutants.
Significantly to decrease the emission of hydrocarbons (HC) and carbon moNOxide (CO), particulate matter (PM), and nitrogen oxides (NOx), exhaust gas after treatment with oxidation catalysts and de-NOx catalysts must be applied. This requires, however, low-sulfur levels in the fuel so as not to poison the catalysts. Low aromatics levels are also environmentally advantageous.3
A fuel that meets those requirements is the synthetic fuel made from natural gas with the Fischer-Tropsch process using cobalt-based catalysts, such as from the Cherry Point pilot plant.
If iron-based Fischer-Tropsch catalysts are used, higher levels of olefins and, under certain conditions, aromatics are produced that require additional expenditures for their removal.
To make Syntroleum S-2 synthetic fuel (a diesel-range fuel produced from Fischer-Tropsch synthetic crude), only mild hydrotreating is required.
To maximize fuel production, the high-molecular-weight hydrocarbons produced can be cracked into fuel-range molecules. Table 1 presents the properties of Syntroleum S-2 fuel, EPA No. 2 fuel, and diesel-fuel requirements, according to current US and European specifications.4 5
Several fundamental differences between synthetic fuel (S-2) and conventional fuel (EPA No. 2) exist.
The first and probably the most important difference is that synthetic fuel contains essentially no sulfur and no aromatics. Because Syntroleum S-2 fuel is almost hydrogen saturated and consists of more than 99% paraffins, the cetane index (CI) is exceptionally high.
Because paraffins have slightly lower specific gravities vs. aromatic molecules with similar carbon number, the API gravity for the synthetic fuel is higher than the values generally obtained for conventional fuels. The higher API gravity results in lower volumetric heating values but higher heating values on a weight basis.
Numerous studies have evaluated emissions from conventional, unmodified diesel engines using a variety of synthetic fuels. Table 2 shows results from the work done at Southwest Research Institute, San Antonio, for a comparison of Syntroleum S-2 with EPA No. 2 fuel using an unmodified heavy-duty 5.9-l. Cummins engine on a chassis dynamometer.5
As can be seen, the synthetic fuel has significantly lower emission rates than the conventional fuel without engine modifications or exhaust gas after treatment. Especially, the 46% lower particulate matter emission is notable. Other tests performed showed similar results.
The lower emission rates can be explained as following:
- Lowering the sulfur content decreases the amount of sulfate particles in the exhaust gas resulting in an almost linear decrease of PM with decreasing sulfur content
- Increasing the cetane number (CN) and decreasing the aromatics content improve fuel combustion and tend to lower NOx and PM emissions.6
The use of low sulfur, low-aromatics fuel lowers the emission rates and opens the way to utilize effective exhaust gas after treatment systems to meet future emission limits.
Specialty products
In addition to the synthetic fuels sector, Fischer-Tropsch liquids can be used in the synthetic lubricants and specialty-chemicals sector.
Depending on the planned utilization of Fischer-Tropsch liquids, the applied processing steps vary significantly. The processing steps are basically conventional technology. In any case, in the use of cobalt-based catalysts, the derived final products are of exceptionally high quality because of the lack of sulfur and aromatics in Fischer-Tropsch liquids.
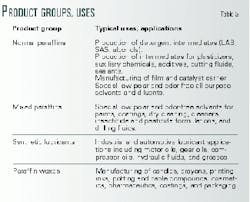
Table 3 summarizes the main product groups and their typical uses and applications.
Table 4 shows typical physical properties for a normal paraffin. This normal paraffin cut in the C10-C13 carbon-number range is a typical feedstock for the production of linear alkyl benzenes (LAB), an important detergent intermediate.
The high purity and the nondetectable aromatics and sulfur content of normal paraffins produced via Fischer-Tropsch synthesis are advantageous for many other applications as well.
Another use of Fischer-Tropsch liquids is the production of synthetic lubricants. This water-clear lubricant has a high viscosity index (VI) and low volatility and can be used as high-performance lubricant base fluid.
Sweetwater plant
In November 1999, Tessag signed a project-development agreement with Syntroleum Sweetwater Operations Ltd. to provide a fixed price for the design and construction of the first commercial-scale application of Syntroleum's GTL technology, known as Sweetwater project.
Since inception, the plant's refining section has expanded to include more products than originally anticipated.
This grassroots plant, expected to cost more than $500 million, is designed to produce 10,000 b/d of liquid specialty products: synthetic lubricants, synthetic paraffins, and other high-value specialty chemicals.
A site has been selected about 4 km from the North West Shelf LNG plant on the Burrup Peninsula of Western Australia. Agreement for the natural gas supply required to operate the plant has been signed with North West Shelf Joint Venture Partners. Most of the permitting for the plant has been obtained and the project awaits a firm lumpsum engineering, procurement, and construction contract with Tessag before debt and equity to fund the project can be finalized.
The Western Australia state government has agreed to invest more than $30 million (Australian) in infrastructure, including roadways and desalination facilities. Syntroleum expects construction to begin in this year with the plant beginning operations in 2004.
The company is receiving additional support from the Commonwealth of Australia, which recently became a licensee of the Syntroleum process.
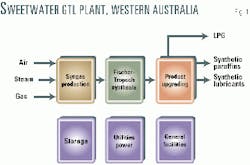
The Sweetwater plant can be structured in three main areas (Fig. 1):
- Syngas production.
- Fischer-Tropsch synthesis
- Product upgrading. Additional areas exist for storage, utilities, power generation, and general facilities.
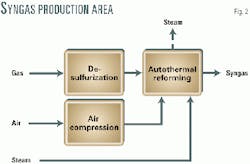
Syngas production is based on Syntroleum's autothermal reforming technology (ATR). Fig. 2 shows the simplified arrangement.
Natural gas is preheated, desulfurized, and then fed together with preheated compressed air and steam to the ATR. The hot syngas leaving the ATR is quenched and further cooled. No air separation nor CO2 removal is required.
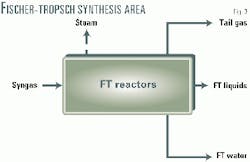
The Fischer-Tropsch synthesis area consists of a Fischer-Tropsch reactor section and the downstream Fischer-Tropsch liquids recovery (Fig. 3). A multitubular fixed-bed reactor design was chosen for the Fischer-Tropsch synthesis because this design, combined with the catalyst system designed for the project, produces a high yield of the desired products.
The plant is designed for a once-through operation using Syntroleum's Fischer-Tropsch catalyst. Therefore no recycle or recompression is required.
Fischer-Tropsch liquids, Fischer-Tropsch water, and low-btu tail gas leave the Fischer-Tropsch synthesis area. The low-btu tail gas is used as fuel in the process to enhance the overall efficiency. Fischer-Tropsch water is treated in the utilities area for further use.
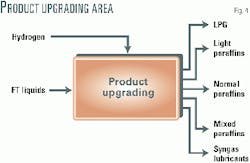
The Sweetwater plant includes a product-upgrading area necessary to produce the targeted specialty products (Fig. 4). The upgrading area includes hydroisomerization technology, licensed from the licensing division of Lyondell Chemicals Co., and paraffins-separation technology licensed from UOP.
The predominant products from this area will be synthetic lubricants. Further products will be LPG, light paraffins, normal paraffins, and mixed paraffins.
References
- Christensen, T.S., Christensen, P.S., Dydkjaer, I., Hansen, J.H. Bak, and Primdahl, I.I., Stud. Surf. Sci. Catal., 119, 883, (1998).
- Espinoza, R.L., Steynberg, A.P., Jager, B., and Vosloo, A.C., Applied Catalysis A: General, 186, 13, (1999).
- Concawe, Report No. 99/62, Concawe, Brussels, Belgium, September 1999.
- Snyder, P.V., Russell, B.J., Schubert, P.F., Syntroleum Publication at Clean Fuels 2000 Meeting, San Diego, Feb. 7-9, 2000.
- Directive 98/70/EC of the European Parliament and of the Council, October 1998.
- Chevron, Diesel Fuels Technical Review (Fischer-Tropsch R-2), Chevron, San Francisco, 1998.
The authors
Paul Schubert is vice-president of research and development for Syntroleum Corp., Tulsa. Previously, he served as vice-president of instrumentation with Monitor Labs Inc., vice-president with Catalytica Inc., senior chemist on the catalyst manufacturing development team with Phillips Petroleum Co., and senior research chemist with Engelhard Corp.
Schubert holds a BS in chemistry from the University of Arkansas, a PhD in inorganic chemistry from the University of Illinois, and has pursued graduate studies in business administration at San Jose State University, San Jose, Calif.
C. A. Bayens is vice-president of engineering for Syntroleum Corp. Previously, he has served as president of Shell Synthetic Fuels Inc. He holds a PhD in chemical engineering from Johns Hopkins University, Baltimore.
Larry Weick is vice-president for licensing and business development for Syntroleum Corp. Previously, he was an independent consultant and for 12 years worked for ARCO in positions in finance, planning, and business development and also served as a director for ARCO. Weick holds a BS in electrical engineering from the University of Nebraska and an MS in engineering economics from Stanford University, Stanford, Calif.