Composition of recycle oil helps determine coke yield
Knowing the composition of the recycle oil charged to the coker fractionator helps determine coke yield and thus coking economics.
The composition of recycle oil is not easy to determine, however, because it does not exist separately from the coke-drum product.
A method proposed by Zhejiang University, China, determines the composition and coke yield of recycle oil found in delayed coking. To accomplish this, the method estimates the subfraction content and cut fraction distribution of the coking feedstock. That is, it estimates the content of the heavier hydrocarbons that come off the bottom of the fractionator to be recycled to the furnace.
To show the effect of throughput ratio (TPR) on coke yield, coking experiments of feedstock with varied TPR were also conducted.
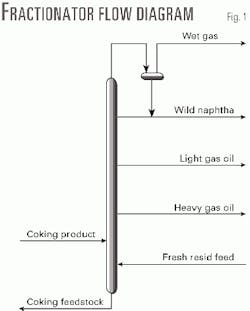
Recycle oil is the heaviest part of product of the coking reactors. It is mixed with fresh resid feed at the bottom of the fractionator and charged to the coker furnace. Fig. 1 shows the relationship among the fresh resid feed, coking product (with recycle oil), and coking feedstock (furnace charge).
The unit's TPR is defined as the volumetric ratio of furnace charge to fresh resid feed. It can also be considered a mass ratio because the density of the furnace feedstock and the fresh resid is almost equal. The furnace charge consists of two inseparable sections: fresh resid feed and recycle oil.
Refineries can use TPR to control liquid yield, heavy coker gas oil specifications, shot make, and cost. Although the volume of recycle oil is easily determined, its composition, which is useful for choosing the proper TPR, cannot be analyzed alone.1
Content of recycle oil
Several key variables affect delayed coking yields and economics. These include resid feedstock quality and three operating parameters: furnace-outlet temperature, TPR, and coke-drum pressure.1
In this experiment, the authors used fresh resid feed and coking feedstock with an initial TPR of 1.37 from China Petrochemical Corp.'s Shengli refinery.
Estimating the contents of the subfractions of recycle oil was the first step to approximating the composition of the recycle oil.
The authors used the SARA3 method to separate the fresh resid feed and coking feedstock into four subfractions: saturates, aromatics, resin, and asphaltene. For a coking feedstock with TPR=1.37, the mass fraction of recycle oil is 0.27, or (1.37-1)/1.37; the mass fraction of fresh resid feed is 0.73, or 1-0.27.
The authors used ai to express the ith mass fraction of coking feedstock and bi to represent fresh resid feed.
Thus, the mass fraction of the ith subfraction of recycle oil is (ai-0.73bi)/0.27.
Table 1 shows the main subfractions of recycle oil are saturates and aromatics. The cause of the negative value of asphaltene, which is very small and acceptable, may be experimenal deviation. The results are in agreement with literature,4 which has reported that recycle oil is rich in aromatics.
The SARA method gave a useful estimation of the composition of the recycle oil. For the coking feedstock used in this article, recycle oil is mainly composed of 57% saturates and 42% aromatics.
Yield from recycle oil
The authors estimated the cut fraction distribution from the data of fresh resid feed and coking feedstock by using gas chromatography simulated true boiling point (TBP) distillation. Narrow cut was determined with a boiling point range of 30 degrees C. The steps are as follows:
The mass fraction of ith narrow cut of recycle oil is ci-o.73di. In the expression, ci expresses mass fraction of the ith narrow cut of coking feedstock, and di is the ith narrow cut of fresh resid feed.
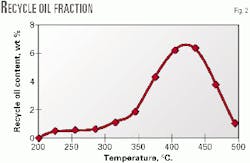
Fig. 2 shows the results of this distillation. For the coking feedstock (TPR=1.37) used in this article, the cut fraction lies between 350 degrees C. and 480 degrees C. and reaches a maximum yield near 430 degrees C., which is useful for the temperature design and operation of a delayed coking unit.
TPR's effect on coke yield
To show the effect of TPR on the coke yield, the authors conducted coking experiments in a small batch reactor at 460 degrees C. The authors varied TPR (1.0, 1.1, 1.2, 1.3, 1.37) by adding fresh resid feed to the reactor.
The reactor was a stainless steel tube (25 cm in diameter and 2.5 cm thick), heated by tin bath. A controller controlled the temperature of the reaction.
The coking reaction was complete when the amount of gas produced was less than 2 ml/10 min. Coke yield was calculated based on the mass of the carbon disulphide (CS2)-insoluble fraction. A complete reaction took 8-10 hr.
Equation 1 (see box) describes the relationship among TPR, added fresh resid, and the coking feedstock (1.37). Note that if M2 is zero, TPR is 1.37.
Equation 2, derived from Equation 1, shows the mass ratio of the coking feedstock to the fresh resid added.
Two sources contribute to coke formation: fresh resid feed and recycle oil. Since the fresh resid feed and the recycle oil are inseparable, there is no way to determine the amount of coke produced by each part. Thus, the authors define relative coke yield of recycle oil as shown in Equation 3.
Equation 3 assumes that coke yield from the fresh resid feed, yp, is constant. It is determined by the coking reaction of fresh resid feed alone.
In Equation 3, y is the coke yield based on experimental feedstock. It is the mass of coke divided by the mass of the experimental feedstock. The v is the mass fraction of fresh resid feed in the experimental feedstock.
The numerator of Equation 3, y-ypv, represents the coke mass produced by the recycle oil, and the denominator, r, is the mass fraction of recycle oil in the experimental feedstock. By definition, v+r=1 because v and r are components of the experimental feedstock.
Table 2 shows that, based on fresh resid feed, coke yield (yv) increases as TPR increases. This yv is the coke yield based on fresh resid feed, or y divided by v.
Although it is known that recycle oil also produces coke, the coke yield of the experimental feedstock (y) and the relative coke yield of the recycle oil (e) decrease as TPR increases.
Thus, coke yield is not the simple summary of the coke yields of fresh resid feed and recycle oil. Similar results have been reported.5
Recycle oil may change the thermal solubility of fresh resid feed and vary coke yield. Former research has shown that thermal solubility and feedstock composition have significant impacts on coke yield.6 7
References
- Stefani, A., Hydrocarbon Processing, June 1996, pp. 99-103.
- Kelton, G.P., Torres, D.L., and Rawlins, H., Hydrocarbon Processing, March 1998, pp. 111-16.
- Xu, Yuming and Cheng, Zhiguang, Acta Pet. Sin. (Pet. Process. Sect.), Vol. 13, No. 3, 1985, pp. 13-22.
- Mochida Sao, Furuno Tetsuya, Korai Yozo , et al., OGJ, Vol. 85, No. 5, 1986, pp. 51-56.
- Yang, Jitao, Chen, Jinrong, Sun, Zaichun, et al., Acta Pet. Sin.(Pet. Process. Sect.), Vol. 10, No. 2, 1994, pp. 1-11.
- Que, Guohe, and Liang, Wenjie, Vol. 71, Fuel 1992, pp. 1843-45.
- Lott, R., 13th World Petroleum Congress, Vol. 18, No. 7, 1991.
The authors
Li Xinxue is a doctoral postgraduate in the chemical department of Zhejiang University, Hangzhou City, Zhejiang province, China. He has 6 years' experience in petroleum refining. He holds a BS in petroleum refining engineering from Daqing Petroleum Institute and an MS in chemical engineering from the Petroleum University of China.
Lin Ruisen is a professor in the chemical department of Zhejiang University. His works involves research on petroleum chemistry and thermochemistry. He holds a BS in chemistry from Xiamen University, China.