Molecular gate removes nitrogen from gas
A new process, using a patented adsorbent material, recovers nitrogen from natural gas at locations that previously were uneconomical because of their low producing rates, says developer Engelhard Corp., Iselin, NJ.
Engelhard says its process, called a "molecular gate," is economical at flow rates as low as 5 MMcfd.
First field test
Engelhard installed its first "semicommercial" molecular gate in August 2000 on a Tom Brown Inc. lease in the Hamilton Creek field, San Miguel County, Colo. (Fig. 1).
The system is designed to reduce the nitrogen content in the natural gas to a pipeline nitrogen specification of 3-5% from the 15-18% produced from the well.
According to Engelhard, the Gas Technology Institute (GTI) has estimated that about 10-15% of the natural gas reserves in the US contain nitrogen that must be removed before the gas can meet pipeline specifications.
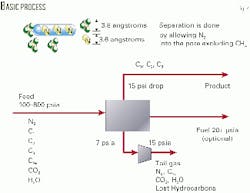
Molecular gates
Engelhard describes molecular gate technology as a process that traps nitrogen molecules in a fixed bed of adsorbent while allowing methane to pass through at feed pressure.
Its adsorbent is unique in that the adsorbent pore size can be adjusted to remove target molecules selectively.
Existing adsorbents have fixed pore openings, such as 3, 4, 5, or 10 Å, and Engelhard indicates these are ineffective in separating nitrogen from methane because the molecule sizes do not fall on either side of the available pore sizes.
Engelhard says it can custom design the pore size of the molecular gate adsorbents to within 0.1 Å.
For nitrogen removal, it uses a 3.7 Å pore size to allow nitrogen molecules (3.6 Å ) to enter and be trapped in the adsorbent, while the larger methane molecules (3.8 Å) pass through the adsorbent bed unaffected (Fig. 2).