Tests spot inconsistencies predicting corrosion-inhibitor effectiveness
Laboratory experiments have shown that common techniques for determining corrosion on downhole tubulars give significantly different results.
Weight loss and linear polarization rate measurements differed substantially in the corrosion rates determined as a function of corrosion inhibitor amount on bare steel and precorroded conditions.
The study evaluated corrosion inhibitors' performances in gas wells producing water and gas containing C02 and H2S. It attributed the differences obtained to corrosion layers and to experimental limitations that hindered accurate corrosion measurements.
Corrosion control
One inconvenience in oil and gas production is the large cost of controlling corrosion caused by the fluids being handled. These fluids in many instances contain such contaminants as C02 and small amounts of H2S that in the presence of free water constitute a potential risk for corrosion on metal surfaces.
The combined factors from the various contaminants can cause a variety of corrosion-related phenomena, such as pitting, stress corrosion, and hydrogen-induced cracking. Therefore, determination of gas stream corrosivity is important to control internal corrosion of tubulars and facilities and to establish mitigation programs such as inhibitor treatments.
Despite industry's efforts devoted to study C02 corrosion, little work has addressed the effect of C02 with small amounts of H2S, which is often encountered.1-7 In general, H2S greatly affects the corrosion of steel in the presence of C02.
Recent studies have shown that at certain conditions some steels may suffer from blistering and crack formation probably caused by compaction and the competitive effects at which Fe-S and Fe-C-O scales form during corrosion.7
In general, all the studies cited addressed the effects of bare steel surfaces exposed to the aggressive environment and not to precorroded surfaces that are usually encountered under real operating conditions.
There are many different types of corrosion tests. These include solubility test, bubble test, flow loop, oil-water partitioning, rotating cylinder, high-pressure flow loop, autoclave weight loss, linear polarization resistance, etc. The most sophisticated tests try to address real field conditions by reproducing all aspects of composition, temperature, pressure, and hydrodynamics.
In some instances, some realism is sacrificed for speed, convenience, and costs. This is usually the case when corrosion inhibitor evaluation is carried out with clean surfaces in autoclaves.
Corrosion inhibitor chemistry is based on the formation of either monomolecular or macromolecular films. The films are created with large carbon chain components carried within a solvent that precipitates on the metal surface as an insoluble layer at certain conditions.8
Therefore, the characteristics of the surface, bare or precorroded, are important to the adsorption of active components of the inhibitors that may include alkyl amines, alkyl quaternary amines, alkylethoxyphosphates, imidazolines, etc. The surface condition will somewhat determine the effectiveness of the treatment.
The study discussed in this article examined the corrosion measurement differences found with weight loss and linear polarization resistance techniques, using bare and precorroded surfaces.
Weight-loss measurement
Autoclave weight-loss experiments determined the corrosion rate of API J-55 steel in 3% sodium chloride distilled water at 450 psi (3,103 kPa) of C02 and 0.20 psi (1.37 kPa) of H2S gas at 170° F. (77° C.). The test procedure resembled the commonly used ASTM standard.9
The test used samples machined from bulk material prepared by an external supplier. The samples, after being machined, were degreased in acetone, polished to 600 grit, rinsed with alcohol, stove-dried, weighed, and stored in a dessicator under vacuum.
Each test included three specimens placed for 170 hr in a solution with a volume to coupon surface area ratio of 15 ml/sq cm. Corrosion products were removed with a pickling solution.
Five independent tests were run with the following inhibitor concentrations: 0, 50, 100, 150, and 200 ppm.
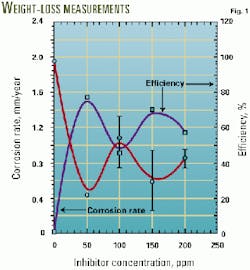
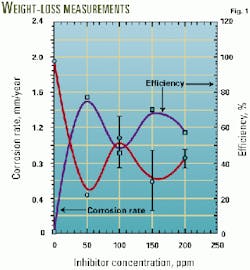
Fig. 1 shows the results in terms of the corrosion rate in mm/year and the efficiency of the product, measured against the condition without any inhibitor added, as a function of concentration for bare steel conditions.
The test without inhibitor established an average corrosion rate of about 1.81 plus or minus 0.21 mm/year. With 50-ppm inhibitor, the corrosion rate decreased to 0.42 plus or minus 0.02 mm/year, establishing an efficiency of 76%, which was the best obtained during the weight-loss measurements. As more inhibitor is added, the corrosion rates tend to increase, especially at 100 ppm.
This behavior is generally exhibited because of a competing effect between the growing corrosion scale and the inhibitor monolayer trying to become attached to prevent scale formation.
This condition has been interpreted as a detergency effect where the inhibitor cleans the surface for the ongoing corrosion processes that continue at the metal-electrolyte interface.12
LPR measurements
The efficiency of the inhibitor was retested with a linear polarization resistance (LPR) technique, but in this case, the test used a precorroded steel surface before the inhibitor was injected into the autoclave (plus or minus 5 mv vs. Ecorr, 0.1 mv/sec).
This procedure, using the same solution, allows for calculating an instantaneous corrosion rate from the linear behavior observed in a typical overvoltage vs. current density plot.10 The slope of the plot is denoted as polarization resistance (Rp), which can be traced over time and is related to corrosion rate.11
In this study, the starting surface condition is bare and corrosion products are intentionally grown and stabilized for a period before the inhibitor is injected into the cell. The test also involved an autoclave vessel but with the appropriate ports for LPR measurements.
The test conditions were the same as in the weight-loss tests.
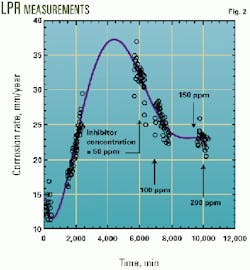
Fig. 2 summarizes the corrosion measurements obtained through the LPR technique as a function of time and inhibitor concentration. It can be readily seen that in the absence of the inhibitor, the corrosion rate increases, establishing a corrosion rate of about 35 mm/year at 6,000 min.
During this time, a corrosion film forms. Once the inhibitor is added (50 ppm), the corrosion rate starts decreasing to 31 mm/year. Further inhibitor additions decrease the corrosion rate to 25 mm/year at 100 ppm, and to 23 mm/year for both 150 and 200 ppm. The best efficiency of 65% is obtained at 200 ppm.
Fig. 3 compares the results from both techniques. The measurements clearly show a difference in corrosion rates. The LPR technique resulted in measuring up to 14 times greater corrosion rates than the weight-loss techniques.
Test results
Weight-loss measurements represent an average rate for a specific exposure period and contain very little kinetic information and probably none concerning long-term exposure or steady-state rates because corrosion is not constant with time (Fig. 2).
This technique is limited because of the relatively small solution volume in the autoclave, which defines a limit for iron concentration, bicarbonate and sulfide concentration, and pH.
This causes the steel to become passive (Fig. 3) from the formation of a corrosion layer that has a composition and protectiveness dependent on the mode of formation. The formation is affected by agitation, rate of temperature increase, pressure fluctuations, C02 and H2S equilibrium, etc.13
This problem could be solved by use of both a larger volume to exposed surface area ratio and a shorter testing time. Some information, however, will be sacrificed in terms of the average corrosion rate obtained and its actual dependence with time.
In terms of the LPR measurements, effective Tafel slopes cannot be measured once oxides have formed. As shown in Fig. 2, the corrosion rate varies with time and hence Rp. It has been pointed out that the assumption of a stable Rp with time is incorrect because this parameter will increase with time rather than stabilize or decrease.13
The Tafel slope extrapolation is masked because inhibitor adsorption is controlled by the electric potential. Likewise, in the presence of a corrosion layer, it is understood that all processes occurring across the solid interface are rate controlled by a combination of Faradaic and non-Faradaic processes as well as the changing environment, both liquid and solid.
The measurement of the Tafel approximation may or may not reflect the actual condition because the inhibitor interferes with the growth or dissolution of the layer but does not affect the Faradaic processes.
For the precorrosion conditions (Fig. 2) before the inhibitor injection, LPR measurements in many instances do not provide protection at the surface by forming a compact iron carbonate scale. This is probably due to the oxide dissolution that may create anodic zones where pitting takes place, accelerating the corrosive process.14
This point is important when one evaluates corrosion inhibitors because many internal corrosion failures in the oil and gas industry are a consequence of pitting.
The dissolution of iron carbonates depends on the temperature at which the steric interaction occurs between the product and the surface to be protected. In this study, the temperature is well below the limit for forming adherent protective layers,1 a fact that reaffirms this observation and explains the relatively low efficiencies obtained.
As discussed, limitations for corrosion inhibitor evaluation include:
- Drawback of evaluating inhibitors through weight-loss measurements because measurements do not take into account all kinetic information.
- Problems that occur because of the limited solution volume.
- Conditions at which corrosion films are grown, which strongly depend on the experimental set up because of the limited solution volume.
- Establishment of a Tafel slope approximation to obtain kinetic information from LPR measurements.
Corrosion inhibitor evaluations are therefore complex and should take into account more real conditions such as flow dynamics and the replenishment of fresh solution.
But weight loss and LPR are relatively simple low cost procedures that are a good approach for a first selection procedure when compared to more-sophisticated, complicated, and time-consuming experimental setups.
In any case, there remains the need for understanding the processes that take place at the solid interphase.
References
- Ikeda, A., Ueda, M., and Mukai, S., "Influence of Environmental Factors on Corrosion in C02 Source Well in Advances in C02," Corrosion, Vol. 2, 1985, pp. 1-22.
- Videm, K., and Kvarekval, J., "Corrosion of Carbon Steel in C02 Saturated Aqueous Solutions Containing Small Amounts of H2S," Corrosion/94.
- Kvarekval, J., "The Influence of Small Amounts of H2S on C02 Corrosion of Iron and Carbon Steel," Eurocorr'97, Trondheim, Norway.
- Ramírez, M., "Efecto de la Relación PH2S/PC02 sobre la Corrosión del Acero ASTM A516," Thesis, Universidad Simón Bolívar, Caracas, 1995.
- Valdes, A., Case, R., Ramírez, M., and Ruiz, A., "The Effect of Small Amounts of H2S on C02 Corrosion of a Carbon Steel," Paper No 98022, Corrosion/98.
- Suárez, M., "Estudio de la Velocidad de Corrosiín en Acero ASTM A515 en Sistemas de C02 con Presencia de Pequeñas Concentraciones de H2S," Thesis, Universidad Central de Venezuela, October 1997.
- Perdomo, J.J., Morales, J.L., Viloria, A., and Lusinchi, A.J., "C02 and H2S Corrosion of API 5L-B and 5L-X52 Grade Steels," Paper No. 00042, NACE International Corrosion Conference, Houston, 2000.
- McMahon, A.J., "The Mechanism of Action of Oleic Imidazoline as an Oil Field Corrosion Inhibitor," Colloids and Surfaces, Vol. 59, 1991, p. 187.
- "Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens," ASTM Standard Practice G 1-90, Annual Book of ASTM Standards Vol. 3, No. 2, 1992, pp. 35-41.
- "Standard Practice for Calculation of Corrosion Rates from Electrochemical Measurements," ASTM Standard Practice G 102-89, Annual Book of ASTM Standards Vol. 3, No. 2, 1992, pp. 406-11.
- EG&G Princeton Applied Research Corp., Application Note CORR1.
- Gutzeit, J., "Corrosion Inhibitor can Cause Corrosion," Materials Performance, July 1993, p. 64.
- Haussler, R.H., "Corrosion Inhibition in the Presence of Corrosion Product Layers," 6th European Symposium on Corrosion Inhibitors, Ferrara, Italy, 1985.
- Gulbrandsen, E., Sundfaer, B., Hesjevik, S.M., Skjerve, S., Nesic, S., and Burchard, T., "Effect of Precorrosion on the Performance of Inhibitors for C02 Corrosion in Carbon Steel," Eurocor Conference, Norway, 1997.
The authors
Jorge J. Perdomo works for the material technology department at Pdvsa E&P, Caracas. His primary responsibilities involve questions regarding corrosion, inhibitor selection, and quality control. Perdomo has a material science degree, specializing in metallurgy, from the University of Simon Bolivar, Caracas and a doctorate in material sciences from the Case Western Reserve University, Cleveland.
Marjorie D. Ramirez is an engineer in the material technology department at Pdvsa E&P, Caracas. She works on investigating corrosion and inhibitors. Ramirez has an engineering degree in materials, specializing in metallurgy, from the University of Simon Bolivar, Caracas.
Alfredo Viloria is a technology manager at Pvdsa's research group Intevep, Caracas. He has worked on various projects concerning materials and corrosion. Viloria has a chemical engineering degree from the Central University of Venezuela, Caracas, and Phd in physical chemistry from the National Polytechnic Institute of Toulouse, France. He is a member of NACE, and Venezuela's Gas Processing Association, National Council of Scientific & Technological Investigation, and Association of Corrosion Engineers.