Successful frac packs require careful planning
Because operators usually pump frac-pack treatments in an offshore environment, a successful job requires extensive planning and preparation.
Frac-pack treatments are difficult completions to perform correctly due to the complexity of having to control many variables during the treatment.
Operators have to be aware of the effect of various additives on fluid performance, as well as the importance of quantifying the fracturing process and pumping a precise treatment according to design parameters developed on site.
Fracturing treatments always have required good logistics and material supply. With frac-pack treatments, however, fluid chemistry as well as treatment procedures have been modified to achieve tip screenouts, develop adequate reservoir fracture geometry, and bypass near wellbore damage.
In addition to bypassing formation damage to enhance production, the treating fluids must also control sand production with a good annular pack and achieve full return of treatment fluids with minimal damage to the reservoir and proppant pack.
Because the mechanics of fracturing unconsolidated formations are different from those encountered in hard rock, operators have placed more emphasis on requiring a short pump in time that averages 30 min/frac.
In these formations, fracturing gel breakdown time need not be as precise because the well will not be placed on production until the workstring is pulled and a production string run. The job design, therefore, should place more emphasis on nondamaging crosslinked fluids.
To meet these requirements, frac gels have become a blend of additives that must be optimized to meet a wide range of well conditions.
Frac packs
Gulf of Mexico well-completion strategies frequently require sand control and gravel pack treatments because they typically have high permeability and porosity, significant drilling and completion damage, and complex mineralogy.
Over the past few years, operators have combined stimulation and gravel packing into a single treatment because of its effectiveness over the life cycle of a well.
Operators usually perform a frac pack during the initial well completion, while the drilling rig is still on location. Treatment timing is important to ensure optimal rig use. This requires significant preplanning beginning with the spud meeting.
After an operator selects a target reservoir for a frac pack, the pumping company will provide a tentative frac-pack proposal with pricing and will assemble necessary data for the final treatment design, such as:
- Casing, tubing, and workstring sizes.
- Sand control tools and screens.
- Data on perforating, zone thickness, well deviation, and reservoir and rock properties.
- Fracturing materials and equipment availability.
- Prejob laboratory tests with chemical lots to be used in the actual treatment.
Laboratory tests provide fluid rheology at reservoir treating conditions for optimizing the breaker, gel, and crosslinker loadings. Also, the lab work tests the proppant according to API procedures for gravel and fracturing proppants, and tests frac fluid compatibility with reservoir fluids and mineralogy.
Further fluid and proppant chemistry optimization can be done on site in the stimulation vessel's laboratory.
After one determines the fluid specifications and finalizes the engineering design, the final job design requires a data frac or minifrac, which will account for all local well variables and provide more-accurate information.
Upon completing the main frac pack, one needs to test the annular pack and restress it to ensure complete packing.
A post-job analysis usually will include an inventory of chemicals and proppants used and a determination of the final frac height and width achieved.
Crosslinked fracturing fluids
The most common frac-pack fluid contains a borate crosslinked guar or derivatized guar polymer, containing enough polymer to attain sufficient viscosity for the frac pack.
Frac-pack fluids typically have a polymer concentration of 25 lb/1,000 gal at temperatures below 150° F. and up to 50 lb/1,000 gal for temperatures above 350° F. A 30 lb/1,000 gal polymer concentration is typical for most Gulf of Mexico wells.
Guar gum is the most common polymer used in frac packs, although in special cases frac packs can contain hydroxypropyl guar (HPG), carboxymethylhydroxypropyl guar (CMHPG), carboxymethyl guar (CMG), and derivatized cellulosics.
Fluid performance depends on its composition. The fluid chemistry can be designed to meet the basic needs of high viscosity, low formation damage, and complete cleanup by varying the polymer and crosslinker content.
Guar has more crosslinking sites available than HPG, and therefore, can yield more apparent viscosity per pound at less cost.
Damage is proportional to the amount of polymer. Less polymer reduces the damage to the fracture system. Laboratory studies indicate that derivatized guars, such as HPG, are as damaging to proppant conductivity as guar. Most pumping companies, therefore, have standardized on guar for crosslinked-system base gel.
Guar and borate systems require a high pH, typically greater than 9.5, to crosslink and support and transport solids. Elevated temperatures require a higher ambient pH value for a proper fluid rheology.
To obtain the desired pH for a specific reservoir treating temperature, pumping companies use different combinations of buffers. Heating will decrease pH; therefore, excess buffer may be required for gel stability at pumping conditions.
The frac design requires a balancing strategy between polymer, crosslinker, and buffer loading to obtain a fracturing fluid sufficiently stable to develop desired fracture geometry and carry solids but yet minimize formation and proppant permeability damage.
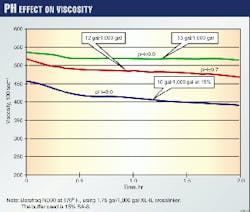
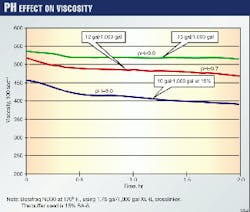
Fig. 1 shows the dramatic effect on the crosslinked viscosity caused by varying amounts of buffer for a given temperature. One observes lower viscosities when the fluid is under buffered. Depending on the fluid design, this could affect the performance and the overall results of the frac-pack treatment.
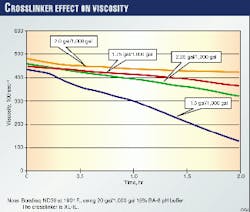
Fig. 2 shows the effect of crosslinker loading on a fluid at a given temperature and buffer concentration. As the crosslinker amount increases, viscosity also increases to an upper limit before decreasing. This behavior also affects job performance. One can control the fluid properties, however, through planned changes during treatment.
The fluid must be designed for both a fracturing treatment and placement of an annular pack with no voids. To accomplish this task, one may have to change the fluid composition during the job, for example, a higher viscosity fracturing fluid followed later in the treatment with a lower viscosity for annular packing. This requires accurate metering and blending of chemicals during the treatment, based on extensive laboratory testing.
In many cases, reservoir bottomhole static temperature is the upper temperature limit; therefore, fracturing fluid can be designed for a lower temperature based on cool-down calculations.
The stability necessary at the lower temperature often reduces the critical chemical loadings for polymer, crosslinker, and buffer and at the same time creates an additional breaking mechanism as the reservoir returns to the bottomhole static temperature.
Clay-control additives
Complex sandstone mineralogy is susceptible to damage from fluids that leak off into the reservoir. This process begins with the drilling fluids and can continue throughout the completion process. This damage is the source of positive skin, which the frac pack is designed to bypass.
Clay-control additives for frac-pack fluids consist of simple salts, simple amines, and clay stabilizers. Some simple salts are potassium chloride (KCl), ammonium chloride (NH4Cl), and calcium chloride (CaCl2).
The industry uses these chemicals extensively in numerous applications to attain compatibility between the treating fluids and the reservoir minerals. Normal concentration required is about 170 lb/1,000 gal (2%).
Some high-permeability formations may require a greater concentration of simple salts because ion exchange by reservoir minerals yields sodium chloride (NaCl) as the treating fluid leaks into the formation. The prevention of formation damage may require a higher concentration of NaCl, usually about 650 lb/1,000 gal (8%).
Simple salts do not provide permanent clay and fines stabilization. Their only benefit is to minimize mineral damage during the time the treating fluid contacts the reservoir. At low concentrations of 2%, simple salts do not adversely affect fracturing-fluid rheology. At concentrations above 5%, simple salts can drastically reduce fracturing-gel rheology and performance.
To provide compatibility with reservoir minerals in hard-rock fracturing where fluid loss is low, the industry uses simple amines, such as tetra methyl ammonium chloride (TMAC) and hexamminediammine. The concentration of these chemicals in the treating fluid is low, about 5 lb/1,000 gal (0.06%), and consequently, they will quickly be depleted from the treating fluid and replaced by NaCl through ion exchange.
This will result in a fluid similar to freshwater and be very damaging to most high-permeability sandstone formations in the Gulf of Mexico. In some cases, these amines can be used in conjunction with simple salts to provide mineral compatibility.
Clay stabilizers are polymeric amines that provide permanent clay and fines protection and thus can minimize damage that can occur during the producing life of a reservoir. These chemicals are added at low concentrations of 10-20 lb/1,000 gal to stabilize the region adjacent to the wellbore and along the fracture faces.
Polymer breakers
After the crosslinked fluid has been placed into the formation, it must be broken to be effectively returned to surface. Three types of chemicals-oxidizers, enzymes, and acids-are used to break the fluid.
In high-permeability formations, the base gel can leak off and penetrate into the formation away from the fracture faces. Thus, a polymer breaker has to provide a break wherever the polymer is. This will minimize damage to the formation, proppant bed, and annulus pack.
Often an acid preflush, pumped ahead of a frac pack to break down perforations, leaks into the reservoir. After a frac pack and during the cleanup phase, low-pH acid mixes with the borate and guar fracturing fluid which then uncrosslinks the gel and helps degrade the guar base gel. An acid preflush with a weak, slow-spending acid, therefore, is common in frac-packs.
Enzymes, which break guar polymers at high pH and temperatures in excess of 200° F., are effective in degrading the base-gel polymer into smaller fragments that are significantly less damaging to the reservoir and proppant permeability. But careful prejob testing should ascertain the degree of enzyme denaturing that occurs from other additives. In many cases, nearly half of the enzyme loading can become useless because of other additives.
Oxidative breakers can degrade guar in temperatures from 130° F. to more than 250° F. Different oxidizing chemicals are available, depending on the temperature and the speed of the break. The chemicals generally used include persulfates, caustics, and hypochlorite bleaches.
All these breakers are water-soluble and stay with the gelled water. They provide the necessary breaking, cleanup, and recovery of the treating fluids. These breakers can be encapsulated or otherwise delayed so that they remain in the fracture with the proppant pack and then are concentrated in the gel filter cake.
The chemicals release after removal of the encapsulation, and one can somewhat vary the time delay.
The various mechanisms provide the industry with flexibility for selecting a combination of chemicals that will adequately break the specific fracturing fluid.
Biocides
Guar and other polymers can act as a food source for bacteria. Bacteria can develop very quickly, especially in the Gulf of Mexico where temperatures are ideal for bacteria reproduction (about 100° F.). Under these conditions, bacteria counts can double every 20 min. Seawater also contains many bacteria types and amounts.
A closed system, such as a fracturing vessel, can experience bacterial contamination. Biocides must be added, therefore, to all water used for preparing guar-based fracturing gels. Biocides will prevent gel degradation and bacterial contamination of the reservoir.
Biocides do not provide instant control of bacteria. Biocides should be added to the water at least 4 hr prior to the preparation of the fracturing gels. Typically, once the biocide is adequately dispersed in the water at the recommended concentration, the bacteria counts should be under control for 24-72 hr, depending on conditions.
Pilot testing of fracturing gels prior to the treatment can identify potential problems from bacteria in the mixing water. A clean gel-mixing system is very important for controlling bacteria on offshore vessels.
Surfactants
Nonionic surfactants can control emulsions in oil wells, lower fluid-surface tension, and aid in fluid recovery in gas wells. Generally, these chemicals are effective at low concentrations and do not cause compatibility problems.
Fluid samples from a well are useful in selecting the proper nonemulsifier and its concentration. In most cases, these additives aid in recovering the frac fluid during the cleanup.
Proppant effects
The primary proppants used in frac packing in the Gulf of Mexico are Econoprop (from Carbo Ceramics Inc., Irving, Tex.) and white frac sand. Many proppants can affect fracturing gel chemistry by altering pH, adsorbing additives and crosslinkers, and changing breaker performance.
Table 1 shows that the addition of 6 lb of proppant added/gal (ppa) can alter gel pH and can change fluid stability. For obtaining the values in Table 1, the Econopro and white frac sand were placed into crosslinked gel and allowed to sit quiescent at 150° F. and 190° F. The pH was measured before pHi and after pHf, the exposure time.
This table indicates that proppants cause slight pH changes, and the direction of the change is dependent on temperature.
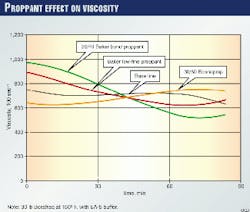
In Fig. 3, one can see the effect of the changes induced by proppant on crosslinked gel rheology. The figure indicates that sand slightly enhances the gel viscosity in the early time, while later, the viscosity falls compared to the control gel. With Econoprop as the proppant, the opposite trend occurs.
During the frac pack, these changes in stability compared to a gel tested without proppant, can result in unexpected problems or additive responses.
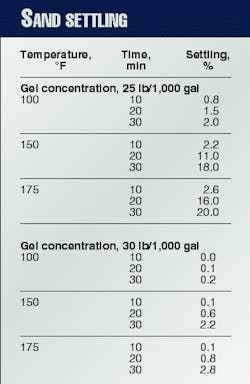
Because the main purpose of the crosslinked fluid is to carry proppant into the fracture in a uniform manner, one needs to pay attention to the polymer fluid's suspension characteristics. Table 2 shows the pronounced effect of polymer loading for two guar concentrations.
In terms of production conductivity, this difference in settling can mean the difference in a completely propped fracture face and a fracture propped mainly at the bottom.
Seawater fracturing fluids
As travel times from rig to shore increase, the need to use seawater for fluids has become necessary to optimize the economics of treating wells in deep water. Seawater can be readily obtained as makeup water for fracturing fluids.
The water, however, must be treated before use. It is very important to filter the seawater to 5 mm or less and add a biocide. The water should then be stored on board to allow the biocide to control bacteria before adding the gelling agent.At high pH, seawater will precipitate some divalent metal hydroxides, such as calcium and magnesium, through a reaction with the high pH buffer. On the average, seawater contains about 1,200 mg/l. of Mg ions and about 400 mg/l. of Ca ions.
In some situations, the amount of solids (0.1-0.2%) may pose no harm to the overall job results. In these cases, a more stable, higher-pH system can be used. In many cases, solids can be damaging to the reservoir and proppant permeability, or the buffer can be depleted at less than its required concentration by its precipitation with the divalent metal ions.
In these cases, the job design should include pH control, so that the precipitates do not form. Additives such as chelating agents can extend the fluid's operating pH range and avoid precipitates, but gel stability can be shortened if too much is added.
In addition to planning the fluid pH for the downhole conditions, the compatibility of seawater with connate reservoir water must also be determined. In many cases, influx of sulfate ions from the seawater can form scale.
If the scale is barium sulfate, the problem can become serious. To alleviate this problem, scale inhibitors may be added to the frac fluids.
Mixing fracturing fluids
Fracturing fluids can be formulated by either batch mixing or on-the-fly mixing.
For a batch-mixed gel, the vessel needs sufficient tanks for transporting the base gel to location, and usually the gel is mixed at the dock prior to sailing.
Advantages of batch-mixed gel include full gel hydration, avoidance of some metering problems, and measurement of the gel viscosity prior to the treatment.
Some disadvantages of batch-mixed gel include mixing more gel than needed, incurring waste-disposal costs, lowering gel shelf life because of bacterial degradation, and incurring higher cost to the operator if the job is cancelled.
To mix fracturing gel on the fly, a vessel must be equipped with accurate metering equipment and a gel hydration tank. On-the-fly mixing allows easier multiple treatments, which result in a more effective use of the frac vessel.
Other advantages of mixing on the fly include handling only the amount of gel required, minimal disposal expense, and avoiding or minimizing bacterial problems and treatment cleanup if the job is cancelled.
Disadvantages of on-the-fly mixing include incomplete gel hydration, especially at very high pump rates, metering problems due to equipment limitations, and measurement of gel viscosity in real-time during pumping.
The industry prefers to mix the frac-pack gels on the fly, whenever possible, and frac vessels are essentially "floating warehouses" with enough supplies to perform multiple treatments before having to return to the dock to restock.
Frac-pack procedures
Frac packs are small, precise fracturing treatments followed by a casing gravel pack. The treatments usually are pumped with gravel-pack tools in the hole.
A crossover tool is the principal mechanical device that allows the fluid pumped down tubing or workstrings to exit into the screen-casing annulus below the packer, generate and fill the fracture, and then gravel pack the casing-screen annulus.
To achieve the precision required to generate a tip screenout, pressures during the treatment and all fluid volumes must be accurately pumped at the designed rate. The pad fluid typically is spotted a few barrels above the packer, with the crossover tool in the circulating position. This operation minimizes bullheading the tubing or workstring volume into the perforations and fracture.
At the start the fracturing treatment, a typical practice is to shift the crossover tool into the squeeze position. Because frac packs take time, the fracturing gel can be crosslinked on a time-delayed basis in the frac string to minimize friction pressure.
Often, the entire pad and slurry will be in the frac string before the treatment reaches the perforations. Therefore, determination of the friction pressure is complicated by this time-delayed crosslinking in the frac string that results in the observed surface pressures during the early treatment stages to have limit value.
Throughout the frac treatment, the pump rate is varied from zero up to the designed frac treatment rate. This requires accurate metering and control of both liquid and dry gel additives. The treatment also requires accurate proppant screws which can meter proppant to the blender tub at low rates (<5 bbl/min) and concentrations (<0.5 ppg) to high rates (20-30 bbl/min) and high proppant loadings (12 ppg).
These are demanding conditions for the frac blender and require special design considerations during equipment manufacturing. In general, these requirements are met by using two sand-screw sizes combined with high-capacity stepping motors.
Quality control
On site quality control is important for all fracturing treatments. Frac jobs involve critical elements that must be continually monitored before, during, and after the job to ensure optimal well productivity.
Critical elements include gel hydration and gel, buffer, crosslinker, breaker, and proppant concentration.
Prior to the job, laboratory testing should define and verify the optimum fluid formulation for the well conditions and frac intentions. A prejob inventory of critical chemicals used during the step-rate test and minifrac can identify problems in delivery rate and/or pressure output.
To identify and correct problems, one can also use real-time monitoring and data acquisition of proppant concentration, additive addition rates, viscosity and pH of base gel, and crosslink time.
During the job itself, one can make visual checks on the crosslinking time and rate of adding the buffers, crosslinkers, breakers, and proppants. This inspection provides assurance that the recorded parameters are functional and accurate.
A comparison of the prejob inventory with the post-job inventory of chemicals is a further check on the treatment. Any discrepancies in chemical and proppant volumes pumped should be included in determining the final frac results and potential production problems.
References
- Ely, John W., Stimulation Engineering Handbook, PennWell, 1994.
- Tiner, R.L., et al., "Frac Packs-State of the Art," Paper No. 36456, October 1996.
- Zheltov, Y.P., McElfresh, Paul, and Kalra, S., "Fracturing Fluids for Arctic Conditions," Neftyanoe Khozyaistvo, March 1999.
- Hall, B.E., and Larkin, S.D., JPT, 1989, p. 526.
The Author
Paul McElfresh is manager of fluids research and development at Baker Oil Tools, Houston. He is involved in developing and characterizing fracturing fluids, acidizing treatments, and cement chemicals.
McElfresh has an extensive background in on site quality control and monitoring of oil and gas well treatments. He holds a BS in chemistry from Texas Tech University and a PhD from Arizona State University.