Study underscores effectiveness of antifoaming agent in DGA sweetening process
Laboratory studies of polysiloxane (DB-31), an antifoaming agent used in diglycolamine (DGA) gas-sweetening units, indicate that DB-31 substantially reduces the DGA solution surface tension and thereby reduces foaming.
The effect is reversed at high concentrations, however, when critical micelle concentration (CMC) is reached.
Nuclear magnetic resonance (NMR) spectroscopy and gel permeation chromatography (GPC) studies showed no degradation or any chemical change of either DB-31 or DGA when exposed to plant temperature and pressure conditions.
This suggests that DB-31 is stable chemically and thus can be recovered.
The research yielding these results follows years of foaming as a persistent problem in Saudi Aramco's high-pressure sweetening plants.
Results of an intensive effort to find the causes and cures of foaming suggested that foaming is caused by contaminants that have accumulated in the system from the feedstock source.
The research also found that plant conditions (high pressure and temperature) alone do not cause DGA to degrade and further proved that such degradation can happen only in the presence of CO2, COS, and CS2.
The change in surface tension of the DGA solution after introduction DB-31 as an antifoam agent is mainly due to a physical change in the DGA solution structure.
DGA advantages
Natural gas in Saudi Aramco fields is soured by H2S and CO2. Before this natural gas can be used, these two souring gases must be removed by a sweetening operation. This acid-gas removal process uses a sweetening agent developed by El Paso Natural Gas Co.1
The process involves the circulation of b, b-hydroxyaminoethyl ether as a treating medium, the commercial name being diglycolamine (DGA).The shortcoming of this process is the tendency of the solution to degrade and form degradation products. This disadvantage of the method can be minimized either by revision of operating variables or by partial reconversion of the degraded solution.
The advantages of using DGA compared to other sweetening agents such as mono ethanolamine (MEA) include the following:
- Removal of H2S and CO2 to 0.25 grains/100 scf of natural gas and CO2 to less than 0.1 mole %.
- Lowered solution rates as compared to MEA.
- Lowered heat requirements for solution regeneration.
- Low chemical losses due to low vapor pressure of DGA.
- Low corrosion rates to common metals
- High stability of solution under operating conditions.2
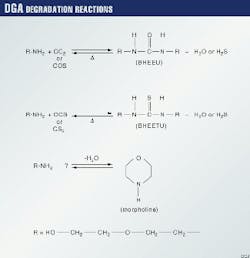
The major chemical degradation product of DGA in gas-treating solutions is N-bis (hydroxyehoxyethyl) urea (BHEEU). It is formed by the reaction of 2 moles of DGA with 1 mole of CO2.
A second degradation product can be formed by the reaction of 1 mole of CS2 or possibly COS with 2 moles of DGA to form N, N-bis (hydroxyethoxy ethyl) thiourea (BHEETU) (3-10).
However, the reactions of CO2, COS, and CS2 with DGA are reversible at temperature ranges of 360-380° F. by hydrolysis in the presence of potassium hydroxide.1
Another possible degradation product is morpholine as a result of DGA hydrolysis. The degradation reaction proceeds according to the equations in the accompanying box.
These degradation reactions cause the abnormal losses of DGA, but foaming represents the most common cause of excessive losses. It also limits the use of the sweetening plant to operating less than its maximum capacity.
An extensive study concluded that foaming is caused by the presence of contaminants produced in the system and less by degradation products mentioned above.11
A treatment of the foaming problem was suggested: An antifoam agent at the level of parts per million is continuously introduced into the system. This treatment was effective. Polysiloxane (DB-31), at a molecular weight of 60,000-100,000 Dalton, was an effective antifoam agent.
The goals of this article are to investigate the role of DB-31 in preventing foaming and to find any type of reaction that DB-31 may undergo at plant conditions. It is also a goal to understand how contaminants cause foaming and the mechanisms by which foaming occurs.
The study involved surface-tension measurements to gain insight into the role of DB-31 as an antifoam agent. Nuclear magnetic resonance (NMR) spectroscopy and gel permeation chromatography (GPC) were used to see whether DB-31 undergoes any degradation reactions under plant conditions of temperature and pressure.
Autoclave experiments
A series of autoclave experiments was designed to load a mixture of 40 wt % DGA solution and the desired concentration of DB-31 in an argon atmosphere.
The first experiment was to test the system, which consists of a PARR PST pressure reactor controlled by a PARR 4843 temperature controller.
This test experiment involved exposing the system to 1,000 psi pressure of argon gas and monitoring it for 24 hr to check for any leak prior to use. A blank sample was first started where 100 ml of 40 wt % DGA solution were exposed to the plant conditions (900 psi and 77° C.; 170° F.). This sample contained no DB-31 and was used as a reference.
DB-31 was then introduced in the system through mixing different amounts of it with 40 wt % DGA solution starting with 200 ppm and stopping at 10,000 ppm. The concentration included 600, 1,000, 3,000, 6,000, 8,000, and 9,000 ppm.
A stirrer maintained the mixing of the system components in the autoclave system. Sampling was done every 2 hr for each experiment, while the pressure and temperature were maintained as mentioned above throughout the course of each experiment.
At last, an experiment was executed in which a larger amount of DB-31 (20 g/l.) was introduced to ensure retrieval of enough DB-31 to perform the desired GPC test analysis. Each experiment lasted 8 hr.
After the system was cooled to room temperature, the gas was vented, and the autoclave residual was used for analysis.
Analysis; instrumentation
Standard solutions of DB-31 in methylene chloride were prepared. The standards covered all the ranges of concentration used in the autoclave experiments and were used as reference in both NMR and GPC analysis.
The residuals of the autoclave experiments after the 8-hr period were extracted with a 5 ml methylene chloride to perform NMR and GPC analysis.
Surface-tension measurements were performed on all the samples obtained after the 2 hr, 4 hr, 6 hr, and 8 hr periods before extraction.
The NMR analysis involved 13C and 29Si spectra on a Varian 400 Unity system, with a 5 mm broad-band probe, in a 51-mm Oxford magnet operating at 9.4 Tesla (94 kilogauss).
Both spectra were proton decoupled. Silicon spectra were acquired at 79.459 Mhz, pulse width was 8.7 ms, transmitter power was 56 db, acquisition time was 0.64 sec, and sweep width was 100,000 hz.
Carbon spectra were acquired at 100.577 Mhz, pulse width was 12 ms, transmitter power was 56 db, acquisition time was 0.64 sec, and sweep width 20,000 hz.
Methylene chloride extracts were mixed 50:50 with deuterated chloroform, and DGA samples were mixed 50:50 with deuterium oxide.
GPC analysis was carried on a 300 x 10 mm TSK 3000 column to measure concentration of DB-31. Molecular-weight distribution was measured on a water HT linear column, 7.8 x 300 mm.
A Varex Mark IV Evaporative Light Scattering Detector was used, with drift tube at 75° C. and exhaust at 48° C. The airflow rate was 62 ml/min. at 48 psi. The pump was an Hitachi L6200A ternary pump with 100% methylene chloride at 1 ml/min. Column pressure was 600 psi under these conditions.
Surface-tension measurements were performed on a precalibrated Surface Tensiomat (Fisher, Model 21) in a manual protocol. The Model 21 uses Du Nouy Ring Method (ASTM-1331).
In a typical experiment, about 25 g of test solution are loaded into a thoroughly cleaned glass vessel that is placed below a platinum-iridium ring. The ring of known dimension is suspended from a counter-balanced horizontal lever-arm of the Tensiomat.
Increasing the tension in the wire raises the arm and the ring, which carries with it a film of the liquid in which it is immersed. The force (dyne-cm) necessary to pull the test ring from the surface film was measured directly as the apparent surface tension of the liquid.
Each measurement was repeated three times and the average of these three readings was recorded.
DB-31's role
The results concluded that DB-31's role as an antifoam agent is physical. This role is related to the surface tension of the DGA solution.
It is possible that the antifoaming role of DB-31 may extend to counteract the effect of the foam promoters if present in the system.
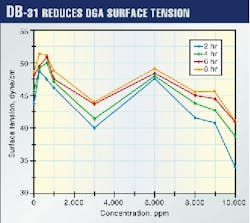
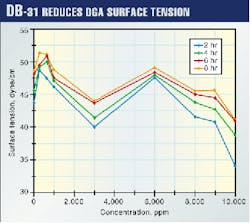
Surface tension of DGA solution was found to increase at small concentration and decrease beyond a certain limit.
This change could be attributed to a slight solubility at low loading of DB-31 in the DGA solution because clear solutions resulted when DGA and low amounts of DB-31 (<6,000 ppm) were exposed to the plant conditions.
Solutions containing higher concentrations (8,000 ppm and greater) of DB-31 in DGA, however, were turbid when exposed to plant temperature and pressure conditions.
Thus, there appears to be a distinct correlation of DB-31 solubility in DGA and subsequent surface tension of the system. A surface-tension inversion was observed when DB-31 solubility in DGA decreased substantially at high (>8,000 ppm) loading. The antifoam behavior of DB-31 in DGA also changed to high foaming around 9,000 ppm loading as was earlier shown.11
The NMR and GPC results showed no sign of any type of degradation neither to the DB-31 nor to the DGA solution as a result of the exposure to the plant conditions.
Surface-tension results
DB-31 reduced the surface tension of the DGA solution. This reduction increased as the concentration of DB-31 increased (Fig. 1).
It is believed to result from a suppression in the surface activity of the liquid induced by changes in the physical structure of the DGA solution. This was concluded because no chemical changes were observed from the spectroscopy results.
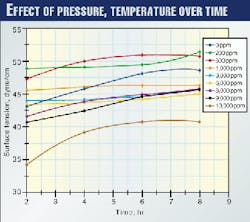
Pressure and temperature seem to counteract the depression in the surface tension of the DGA solution. This is shown in Fig. 2 in which surface tension increases with time. This increase in surface tension is accompanied probably by an increase in the surface activity of the DGA solution. Hence, foaming is likely.
This is better understood because it is known that a low pressure DGA sweetening plant does not exhibit foaming as much as a high-pressure plant does.
NMR results
Both methylene chloride extracts of all the different concentrations used and the remaining DGA solutions were analyzed by both 13C and 29Si NMR spectroscopy. Similarly, both lean-DGA solution and fresh DB-31 were individually analyzed by NMR.
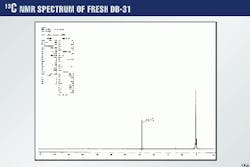
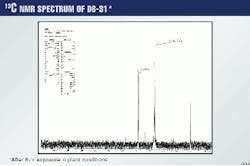
The results were compared to those obtained for the methylene chloride extracts and cooked-DGA solutions. Fig. 3 shows the 13C spectrum of lean DGA solution. In this case, only the carbon resonances are seen.
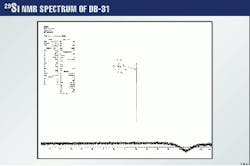
Here again, the silicon resonance is the only one to be seen. The symmetry of the molecule probably reduces the spectrum to a simple representation of one line.
All 29Si spectra of the methylene chloride extracts of all concentrations show no presence of the silicon in them. This is probably because the amounts of silicon present in these samples are beyond the detection limit and could probably be attributed to lower system sensitivity to silicon when compared to carbon.
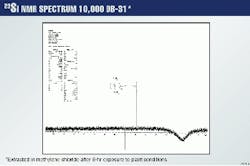
Fig. 5 shows the 29Si spectrum of the highest concentration extract of 10,000 ppm.
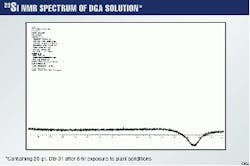
To prove that no chemical change has taken place for both DGA and DB-31, Figs. 6 and 7 show the 29Si spectra for a 20g/l. methylene chloride extract of DB-31 and DGA after they have been exposed for 8 hr to plant conditions of temperature and pressure.
It is clear that the line representing silicon is present only in the methylene chloride extract, which contains the DB-3, but not in the remaining DGA.
The 13C spectra for both DGA and DB-31 are also shown before and after exposure to plant conditions. This further proves that there was no chemical interaction between DGA and DB-31.
Fig. 8 shows the 13C spectrum for DGA after it has been exposed to plant conditions for 8 hr. This figure can be compared to Fig. 3, which is the 13C spectrum of DGA before exposure.
Figs. 9 and 10 show 13C spectra for DB-31 before and after exposure to plant conditions, respectively.
GPC results
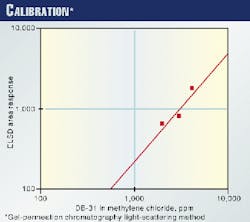
Standard solutions of 1,000, 2,000, 3,000, 5,000, and 10,000 ppm of DB-31 in methylene chloride were used in GPC for calibration. Fig. 1 shows the calibration curve.
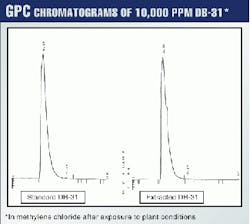
The 20 g/l. extract of DB-31 in methylene chloride was diluted to give 10,000 ppm and was run in GPC to be compared with the 10,000-ppm standard. Fig. 12 shows the GPC chromatograms of both.
As can be seen from this figure, no change in molecular weight of DB-31 occurs after exposure to plant conditions, which again proves no degradation of DB-31 is happening. GPC results also show that a retrieval of more than 90% of DB-31 is possible.12
Additional work
Further study is needed of the mechanism by which DGA hydrolyzes to form morpholine and to determine the thermodynamic behavior of this reaction.
There is also a need to find a quantitative measure of the solubility parameters of DB-31 in DGA under plant conditions to relate the reverse effect (foaming effect) of DB-31 at high loading.
The effect of the known contaminants on the surface tension of DGA solutions should be established to understand its role on foaming in DGA.
And an understanding is needed of why and how known compounds such as carboxylic acids, phenols, etc. cause foaming.
Acknowledgments
Appreciation is due the management of the Saudi Aramco Laboratory Research & Development Center, Dhahran, for their support of this research. Acknowledgment is also extended to G.G. Martinie and Mohammad A-Mahdi for long hours dedicated to performing NMR analysis. And thanks are due Khalil I. Al-Humaiiem and Licerio A. Conanan of LR&DC for performing surface-tension measurements.
References
- Holder, H.L., "Diglycolamine-A Promising New Acid-gas Remover," OGJ, June 2, 1966, p. 83.
- Martin, J.L., Otta, F.D., and Marker, A.E., "Solubility of Hydrogen Sulfide and Carbon Dioxide in a Diglycolamine Solution," Journal of Chemical and Engineering Data, Vol. 23 (1978), No. 2, p. 163ff.
- Moore, T.F., Dingwan, J.C., and Johnson Jr., F.L., "A Review of Current Diglycolamine Agent Gas Treating Applications," Environmental Progress, Vol. 3 (1984), No. 3, p. 207ff.
- Dingman, J.C., and Moore, T.F., "DGA-A Workhorse for Gas Sweetening," Hydrocarbon Processing, Vol. 80 (1982), No. 5, p. 138ff.
- Dingman, J.C., and Moore, T.F., "Compare DGA and MEA Sweetening Methods," Hydrocarbon Processing, Vol. 47 (1968), No. 7, p. 208ff.
- Dingman, J.C., Jackson, J.L., Moore, T.F., and Branson, J.A., "Equilibrium Data for the H2S-CO2-Diglycolamine Agent Water System," 62nd Annual Convention of Gas Processors Association, Mar. 14-16, 1983.
- Freireich, E., and Tennyson, R.N., "Process improves acid gas removal, trims costs, and reduces effluents," OGJ, Aug. 23, 1976, p. 130.
- Hikita, H., Asai, S., Ishikawa, H., and Honda, M., "The Kinetics of Reactions of Carbon Dioxide with Monoisopropanolamine, Diglycolamine, and Ehtylenediamine by a Rapid Mixing Method," Chemical Engineering Journal, Vol. 14, 1972, No. 1, p. 27ff.
- Martin, J.L., Frederick, Otto D., and Marker, E.A., "Solubility of Hydrogen Sulfide and Carbon Dioxide in a Diglycolamine Solution," Journal of Chemical and Engineering Data, Vol. 23 (1978), No. 2, 1978, p. 163.
- Mason, T.R., and Griffith, T.E., "MEA-DGA Switch Saves Steam," OGJ, June 9, 1969, p. 67.
- Harruff, L.G., et al., "Laboratory Study of Shedgum Gas Plant High Pressure DGA Foaming Problem," a report submitted to LR&DC, 1993.
- Martinie, G.D., "Determination of Silicone Antifoam in DGA Gas Sweetening Solutions by Carbon 13 NMR and GPC with Laser Light Scattering Detector," ACS meeting, Bahrain, October 1994.
The Author
Ali M.S. Al-Ghamdi is associate professor of chemistry at King Abdulaziz Military Academy, Riyadh, Saudi Arabia, which he joined in 1987. He holds a BSc (1979) from King Saud University, Riyadh, MSc degrees from Arkansas State University, Jonesboro (1984), and the University of Cincinnati (1985), and a PhD (1987) from the University of Cincinnati. Al-Ghamdi has served visiting appointments at the University of Cincinnati and the University of Akron.