Removing diolefins from coker naphtha necessary before hydrotreating
Syncrude Canada Ltd.'s Edmonton Research centre carried out hydrotreating pilot plant tests with coker naphtha over a typical alumina-based NiMo catalyst to investigate process variables for removal of olefins, diolefins, aromatics, sulfur, and nitrogen.
The removal of diolefins from the coker naphtha before naphtha hydrotreating is essential to making a high quality product.
At high reactor-bed temperatures such as those found in hydrotreating, diolefins tend to polymerize and cause fouling.
Tests were also conducted on increasing the reactor temperature stepwise until the reactor plugged due to polymerization of diolefins. It was found that diolefins and aromatics can be removed at relatively lower reactor temperatures, whereas other reactions occur at higher temperatures.
The reactor was plugged, and the test was ended after 2,000 hr when the reactor bed temperature reached 290° C.
Background
Syncrude operates a surface-mining oilsands plant at the Athabasca oilsands deposit in northern Alberta, and produces Syncrude Sweet Blend (SSB) crude oil from the extracted bitumen. An article on Canada's oilsands industry and detailed Syncrude operations has recently appeared (OGJ, June 28, 1999, p. 44).
The bitumen is currently upgraded in two fluid cokers and an ebullated-bed hydrocracker (LC-Finer). The naphtha, light gas oil (LGO), and heavy gas oil (HGO) products are then hydrotreated to remove sulfur and nitrogen. The hydrotreated products are combined as SSB and shipped to refiners by pipeline.
Unlike conventional crude oils, SSB does not contain any residue. Refiners use the naphtha fraction contained in SSB as reformer feeds, LGO as diesel or jet-fuel blending stock, and HGO as feeds to their fluid catalytic cracking (FCC) units. The quality of FCC feeds from Athabasca oilsands is discussed elsewhere (OGJ, Jan. 19, 1998, p. 43).
In the Syncrude operation, the sources of naphtha are the fluid cokers, LC-Finer, and gas-oil hydrotreaters. The naphtha from the coker, a significant source of naphtha, contains a large amount of sulfur and nitrogen as well as olefins, diolefins, and aromatics.
Diolefins easily polymerize at normal hydrotreating conditions and often foul the reactor. They must therefore be removed before normal hydrotreating.
Commercially, the naphtha is treated in two consecutive reactors: the diolefin reactor and the main reactor. The diolefin reactor is used to reduce diolefins; the main reactor reduces heteroatoms, such as sulfur and nitrogen.
Reducing aromatics and olefins in the main reactor is incidental. In order to optimize unit operation or reactor design, engineers need to understand how sulfur, nitrogen, olefins, diolefins, and aromatics in both reactors are removed at different process conditions.
Pilot plant test results help form operating guidelines for removing diolefins from coker naphtha to alleviate the reactor-plugging problem.
Pilot plant tests
The tests were carried out in a down flow packed-bed reactor (1.7 cm ID and 122 cm overall length) containing 100 ml of commercial 1/8 in. (3 mm) trilobe NiMo catalysts. The catalysts were diluted with the same volume of 46-mesh silicon carbide to minimize flow maldistribution and loaded to a height of 82 cm in the middle of the reactor.
The loaded catalyst was sulfided and precoked (or stabilized) for 1 week using the same naphtha feed. Pure hydrogen was used.
The reactor was heated by three independently controlled electrical furnaces. These furnaces provided a uniform temperature along the length of reactor. The reactor temperature was measured by a movable thermocouple on the outside reactor skin. The average of 12 readings was used as the average bed temperature (ABT).
A detailed description of the reactor and sulfiding method is given elsewhere.1
Two series of tests were performed over the same loaded catalyst with different batches of coker naphtha.
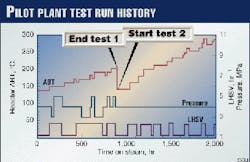
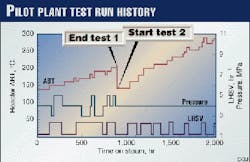
In Test 1, the ABT ranged from 140° C. to 203° C. when reactor pressures were varied among 3, 4, and 5 MPa (or 435, 580, and 725 psig) and liquid hourly space velocities (LHSV) were alternated between 1.2 and 2.4 hr-1. The objective was to investigate how the impurities were reduced at relatively low ABTs.
In Test 2, the pressure was fixed at 4 MPa (580 psig), and LHSV was varied (between 1.2 and 2.4 hr-1). ABT was raised from 140° C. to 200° C. stepwise by 20° C. and above 200° C. by 10° C. until the reactor was plugged due to polymerization of diolefins. It is well known that diolefins polymerize at higher temperatures.
The same ABT was maintained for at least 2 working days at each LHSV. The catalyst bed was plugged at 290° C.
The total run length of both Tests 1 and 2 was 2,000 hr. Fig. 1 shows the run history.
With data from both Tests 1 and 2, correlations to predict product quality were developed as a function of feed properties and process conditions.
The methods by which the samples were analyzed are listed below:
- Density by Anton-Parr density meter Model DMA-48
- Bromine number by electrometric titration
- Diene number by titration using maleic anhydride (UOP method)
- Olefins content by fluorescence indicator analysis (FIA: ASTM D1319)
- Aromatics content by FIA and supercritical fluid chromatography (SFC)
- Sulfur content by Antek sulfur detection 714, oxidative combustion analysis
- Nitrogen content by Dohrman combustion analyzer with chemiluminescent detection.
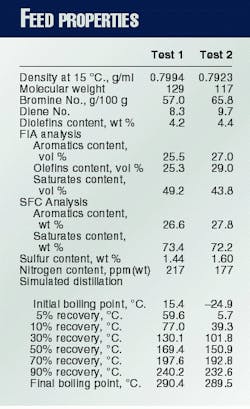
Table 1 summarizes the properties of the naphtha feed. Compared with the feed used for Test 1, the Test 2 feed is lighter (lower density and lower molecular weight) and contains less nitrogen. Test 2, however, contains more olefins, diolefins, aromatics, and sulfur.
Product quality
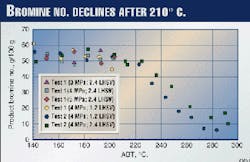
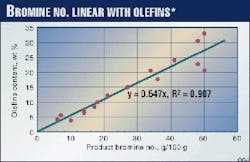
Fig. 2 shows product bromine numbers from Tests 1 and 2. The bromine number has a linear relationship with olefins content determined by fluorescence indicator analysis (FIA), as shown in Fig. 3. The bromine number, which reflects olefins content, is constant below 203° C., but starts to decrease above 210° C. Lower LHSV gives a lower bromine number.
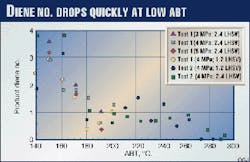
Fig. 4 shows product diene numbers from Tests 1 and 2. Diolefins content is related to diene number and mole wt as shown below:
Diolefins, wt % = (Diene No. x mole wt of naphtha) / (mole wt of iodine)
The mole wt of iodine is 253.84. A typical mole wt of the naphtha product is 120. Therefore, as a rule of thumb, diolefins content (wt %) is about half the diene number. The diene number (or diolefins) drops significantly at low ABT; about 90% reduction is achieved at 180° C. Lower LHSV or higher reactor pressure gives a lower diene number. (Fig. 3 Shown at right)
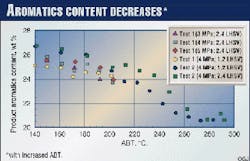
Fig. 5 shows product aromatics content determined by supercritical fluid chromatography (SFC). Unlike the bromine number or olefins, aromatics are hydrogenated at a much lower ABT and decrease as the ABT increases.
It is well known that 380° C. is the approximate optimum ABT for aromatics hydrogenation of diesel cut; that is, the reaction mechanism changes from kinetic control to equilibrium control at 380° C.2
The ABT in the present study was under 290° C., which may be within the kinetic control regime. Higher pressure and lower LHSV also favor the hydrogenation reaction. (Fig. 6 Shown at right)
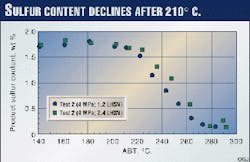
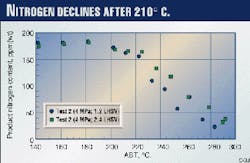
Figs. 6 and 7 show product sulfur and nitrogen contents from Test 2. The trend is the same as the bromine number in Fig. 2. The properties are constant below 203° C. but start to decrease above 210° C. (Fig. 7 Shown at right)
From the above results, it was concluded that only diolefins and aromatics saturation are significant below 203° C., and other reactions are not. Olefins (or bromine number), sulfur, and nitrogen contents start to decrease at 210° C.
The catalyst bed was plugged after 2,000 hr from the startup of Test 1 when the ABT was increased to 290° C. The commercial implication is that diolefins must first be removed at lower ABT before the coker naphtha is hydrotreated at such a high ABT.
Model, correlations, sample calcs
Correlations to predict the extent of hydrogenation of olefins, diolefins, and aromatics, and hydrodesulfurization (HDS) and hydrodenitrogenation (HDN) were developed as a function of the feed properties and operating conditions.
As shown in the equation box, it was assumed that all reactions follow the modified first-order kinetics that include power terms for LHSV and reactor pressure and that the Arrhenius equation applies to the rate constant.
Fig. 8 shows Arrhenius plots of the data. The straight lines in the plots suggest that all reactions can be well expressed by the modified first-order kinetics. The slope of the best-fit lines is E/R (K.-1)
Rearranging Equation 3 in the box, the product diene number (Cp) at specific ABT, LHSV, and reactor pressure (P) can be estimated by the following equation using the parameters in Table 2.
Cp = Cf / exp[exp{ln k0 - (E/R)/(ABT + 273.2) - a ln LHSV +b ln P}]For other properties, the same equation is applied at 4 MPa (or 580 psi) of pressure, where b is zero.Sample calculations to determine product diene number and aromatics content are shown in the box. The results agree well with the pilot plant data in Figs. 4 and 5.
Acknowledgments
The author is grateful to Keng Chung at Syncrude Research for his helpful discussions in preparing this article and to Syncrude Canada Ltd. for its permission to publish it.
References
- Yui, S. "Hydrotreating of Bitumen-Derived Coker Gas Oil: Kinetics of Hydrodesulfurization, Hydrodenitrogenation, and Mild Hydrocracking, and Correlations to Predict Product Yields and Properties," AOSTRA Journal of Research, Vol. 5, 1989, pp. 211-224.
- Yui, S. and Sanford, E. "Kinetics of Aromatics Hydrogenation of Bitumen-Derived Gas Oils," Can. Journal of Chem. Eng., Vol. 69, 1991, pp. 1087-1095.
The Author
Sok Yui is a research associate at the Edmonton Research Centre of Syncrude Canada Ltd. His research involves quality improvement of the products from oilsands bitumen, including hydrotreating, mild hydrocracking, aromatics hydrogenation, and catalyst evaluation.
Before joining Syncrude in 1979, he was section head of manufacturing coordination and planning at Mobil Sekiyu and its refining company, Kyokuto Petroleum Industries in Tokyo, Japan.
He holds BS, MS, and DEng degrees from the University of Tokyo, all in chemical engineering. He is a member of the Canadian Society of Chemical Engineering.