Ethylene oxide successfully removed from overturned tank car
By performing a hot tap and using special tools to defeat safety and check valves, railroad and chemical plant personnel safely removed ethylene oxide from an overturned tank car in January 1998.
The tank car flipped onto its side when it was hit by a train traveling at 35 mph. The oncoming train had just moved onto a shorter side track from a main line into a southern chemical plant.
Although severely damaged, the tank car carrying ethylene oxide, did not rupture or leak.
Ethylene oxide is toxic and extremely flammable. At room temperature, it must be maintained under pressure to remain in a liquid state. At atmospheric pressure, it boils at 51° F.
On-site personnel acted immediately to evaluate the potential hazard. They set steps in motion to protect the public, the facility, and the environment in the event a leak occurred.
Tank car details
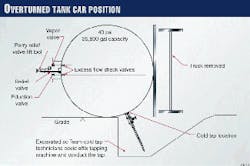
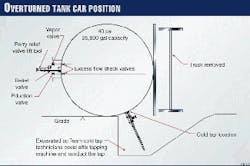
The tank car that contained ethylene oxide was 40 ft long and 10 ft in diameter. The shell of the tank car was constructed of carbon steel (TC-128-B) and had 4 in. of external thermal insulation. This insulation helped the tank car to maintain a constant internal temperature and provided some protection from explosion, should there be a fire on the outside of the tank car.
The tank car also had an internal Pla site lining, which is a sprayed lining of vinyl ester, to protect the tank from any corrosive contents.
Several safety features had been built-in to the design of the tank car. The shell was 9/16-in. thick and had been initially pressure-tested at 300 psig. A standard relief valve (set at 75 psig), two liquid eduction valves, and one vapor valve were undamaged.
These valves were inside the protective housing on top of the tank car. With the tank car laying on it side, the valves were now horizontal.
Fig. 1 illustrates the position of the car and the location of various valves.
Excess-flow check valves between the eduction and vapor valves and the tank contents provided another added measure of safety. These check valves make the eduction and vapor valves inoperable if the tank car is not upright.
Alternative solutions
On-site company, railroad, and contract emergency-response officials concluded in their initial meeting that no effort should be made to move the car until the contents were removed and stored.
The overturned car contained 26,000 gal of liquid ethylene oxide at 40 psig. The liquid would have to be removed to an alternate storage tank before the car could be safely moved.
With the car lying on its side, none of the car's existing valves could be used to empty the tank in the usual manner. The check valves could only be opened with a special excess-flow valve defeater tool attached to the eduction or vapor valve.
Team Industrial Services Inc., Alvin, Tex., a contractor specializing in emergency-response hot taps and on-line leak-sealing services, suggested that at least half of the ethylene oxide could be removed through the relief valve with the company's Perry relief-valve lift tool.
The Perry valve tool compresses a relief valve's spring to hold the valve open. This would allow transfer of the tank car's contents.
With this tool and with the car on its side, only 50% of the contents could be transferred from the tank through the relief valve. The product remaining in the bottom half of the tank car still would have to be removed.
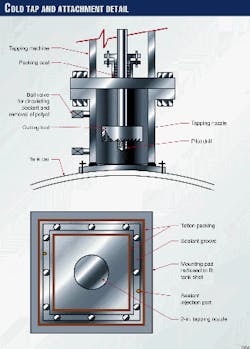
A newly attached nozzle at the lowest point on the tank car's shell with a cold tap could remove the remaining contents.
A cold tap is similar to a hot tap except that a nozzle is bolted to the car (rather than welded) and the cutting tool is flooded with coolant while the tap is being performed. Many cold taps have been performed on flammable services that would otherwise be considered too hazardous to attempt.
Here, a cold tap would gain access through the tank wall. Contents then could be drained off in the liquid state. The nozzle would be attached by drilling and tapping blind bolt holes into the tank wall.
As officials discussed the possibilities, four options emerged:
- Attach the nozzle to the bottom of the tank and perform the cold tap. Drain the ethylene oxide off through the bottom nozzle to storage. The concern, however, was the tap might generate enough heat to ignite the ethylene oxide.
- Connect a line from one of the tank car's existing valves to a flare. Then, flare the entire tank contents as a vapor.
- Inject a polyol material that is heavier than ethylene oxide into the tank car through a product valve while removing the ethylene oxide as a liquid through the relief valve. The polyol material is not nearly as toxic, volatile, or flammable as the ethylene oxide. And, because it is heavier than ethylene oxide, the polyol would float the ethylene oxide up and out the relief valve. Some mixing of the polyol with ethylene oxide would occur.
- Inject polyol material into the tank car until enough ethylene oxide is removed through the relief valve. When the ethylene oxide has been removed, excavate a workspace beneath the tank car, cold tap the bottom of the tank, and remove the contaminated polyol through the tapped nozzle.
Proposal No. 4, a combination of Nos. 1 and 3, was agreed upon as the safest and best way to remove the ethylene oxide and polyol from the tank car.
Detailed steps
It was decided that the ethylene oxide would be drawn off through the tank car's safety relief valve with the Perry relief-valve lift tool. The polyol would be pumped in through one of the tank car's eduction valves to raise the ethylene oxide up and out through the relief valve.
With the conceptual plan complete, the team focused its attention on details of manufacturing, sealant, and construction materials for the cold tap. Items addressed include the following:
- The injected sealant material to seal the joint between the tapping nozzle and the tank shell would have to be chemically compatible with ethylene oxide.
- The steel cuttings produced by the tapping cutter would have to be chemically compatible with ethylene oxide.
- The best method (which valve to use) for extracting the ethylene oxide while pumping in the heavier polyol would be reviewed again.
- The tank car's contents would be kept as cool as possible to minimize pressure increases inside.
- Because ethylene oxide breaks down with elevated temperatures yielding oxygen, which is dangerous in the presence of flammable material, a demonstration of the cold tap would be performed on a section of 1/2-in. thick rolled carbon steel plate to measure the resulting temperature that would develop on the shell.
- A Team Industrial Services technician would install the tapping nozzle and perform the cold tap. He would work alone in the excavation beneath the car wearing a protective chemical suit with supplied breathing air and a lifeline in the event of an emergency.
A second technician would stand by as a safety watch, and a third would tend the first technician's air bottles to provide uninterrupted breathing air.
The sealant material was verified to be compatible.
Steel cuttings were tested in contact with ethylene oxide and found to be compatible.
An awning was erected over the tank car to keep it shaded and cool.
A trial cold tap was performed on metal the same thickness as the tank car's skin. The resulting temperatures that developed were found to be acceptable.
Holes then were drilled, tapped, and fitted with studs the exact depth they would be drilled on the tank car's shell. Stud strength and stability were tested and found to be satisfactory.
The plan was set in motion.
The Perry valve tool was attached to the safety relief valve. The valve was opened and ethylene oxide transferred to another tank car 100 ft away. Polyol was injected to replace the material drawn off and to float the remaining ethylene oxide up and away from the tank bottom where the cold tap was to be performed.
Within 24 hr, the ethylene oxide was transferred to storage.
Work began immediately to attach the tapping nozzle for the removal of the polyol remaining inside the car. Fig. 2 shows the details of the cold tap.
Blind holes were drilled and tapped into the tank car's shell at the tank's lowest point, and the nozzle was bolted up. Sealant was injected between the nozzle and the shell and allowed to cure. The tapping machine and a new ball valve were then bolted up to the tapping nozzle. A pressure test using nitrogen verified the assembly was leak free.
To prevent any heat build-up, polyol was pumped as a coolant into the tapping assembly via the attached ball valve during the tapping operation. The tap was completed within an hour without incident or problems.
The contaminated polyol material then was removed successfully from the tank through the ball valve.
Standard salvage operations at the site began shortly afterward. The car was filled and flushed with water twice. The third time it was filled, the tank car structurally failed. The water was removed and the salvage was completed.
The Authors
Jim Pennington is a consultant for Team Industrial Services Inc. on matters pertaining to on-line leaks. Previously, he worked as the engineering and quality assurance manager at Team for 22 years.
Pennington holds a BS in engineering from Texas A&I College, Kingsville, Tex.
Vergel Perry is corporate hazardous material response manager, Freeport, Tex, for Garner Environmental Services, Deer Park, Tex. He is responsible for branch offices in Fort Worth, San Antonio, and the Louisiana state police/Garner has-mat training facility in Holden, La.
Previously, Perry worked for Dow Chemical Co., Freeport, Tex. At Dow, he served as a training supervisor, safety supervisor, safety director, chief of fire and security at Oyster Creek Division, and emergency response coordinator for Dow's Texas operations. He holds a BS in industrial arts from Southwest State University, Marshall, Minn.
Patrick Brady is manager of hazardous materials field operations and emergency response for Burlington Northern and Santa Fe Railway, Fort Worth. He started with Burlingon Northern Railroad in 1991 as a corporate industrial hygienist.
In 1992, Brady became the assistant director of hazardous materials safety. Upon Burlington's merger with Santa Fe in January 1996, he assumed his current position.
Brady is a certified safety professional. He holds a BS in enviromental and public health from the University of Wisconsin, Eau Claire.