Plant data, modeling validate hybrid solvent for mercaptans removal
Peter A. Wissner
Jack D. McJannett
Dow Chemical Canada ULC
Calgary
Andrew J. Lemonds
Dow Chemical Co.
Freeport, Tex.
David Majid
Spectra Energy Corp.
Fort St. John, BC
New modeling tools delivered excellent predictions of removal rates of H2S, CO2, and mercaptans along with total acid gas volumes.1 Simulation studies noted particularly strong agreement in characterizing the performance of aged solvent.
Operating data from Spectra Energy Corp.'s Jedney processing plant in Fort St. John, BC, have revealed long-term performance results of a customized hybrid solvent used to treat the simultaneous presence of acid gases and trace-sulfur compounds (e.g., mercaptans) in unconventional natural gas.
While raw natural gas streams containing H2S and CO2 are commonplace and often easily processed to meet pipeline sales-gas specifications using amine-only solvents, treatment becomes more difficult and process selection more complex when high levels of mercaptans also are present.
Comprised of an amine, a physical solvent, and water, hybrid solvents remove acid gases (H2S, CO2) and mercaptans (e.g., methyl, ethyl, and higher mercaptans) from raw gas streams. The amine reacts with and removes H2S and CO2 (acid-base reaction), while the physical solvent removes mercaptans by solubility. This hybrid solvent can remove more mercaptans than an amine solvent alone.
Hybrid solvents, however, typically require higher solvent-circulation rates than amine-only solvents. The physical solvent also will absorb more hydrocarbons than the amine, leaving more unwanted hydrocarbon in the rich solvent.
Achieving plant optimization with regard to acid gas and mercaptans removal, undesired hydrocarbon coadsoprtion, circulation rate, and energy consumption when using a hybrid solvent requires balancing the relative compositions of the solvent's three components.
This article details the Jedney plant's implementation of Dow Chemical Co.'s UCARSOL HYBRID-703 solvent to address these challenges, presenting historical plant data as well as comparisons with subsequent process simulation results.
Plant overview
Located in northern Canada in ambient temperatures ranging from -40° C. (winter) to 30° C. (summer), the Jedney plant processes gas containing 2.1 mol % H2S, 3.1 mol % CO2, and 300 ppmv mercaptans (predominantly methyl and ethyl mercaptans) delivered via a combination of pipeline sources.
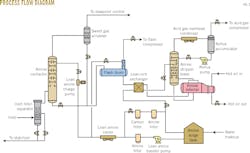
Based on an initial design selection concluding that a hybrid-solvent, sour-gas treating unit coupled with sweet-gas refrigeration (for hydrocarbon and water-dewpoint control) was most cost effective,2 Spectra configured the plant's two 78-MMcfd processing trains (J1, J2) with Dow's hybrid solvent for simultaneous removal of H2S, CO2, and mercaptans (Fig. 1). This approach achieved treated gas specifications of 6-mg/cu m maximum H2S content, <2 mol % CO2 content.After passing through the refrigeration unit, which also removes mercaptan, combined processed sales gas from the J1 and J2 amine contains a total sulfur content of <23 mg/cu m.
Built with the ability to shift the rate of feed gas via an interconnect pipe to manage variability in gas composition, the Jedney plant's J1 and J2 processing trains are equipped with the following:
• Inlet separator, coalescing filter.
• Absorber with treated-gas scrubber to catch entrained amine solution (30 two-pass trays total, with lean-amine feed points to trays 20, 25, and 30).
• Flash tank with flash-gas absorber (for compression and recycling of flash gas).
• Lean-rich heat exchanger (plate-and-frame).
• Regenerator (20 trays total, including 18 stripping trays and two water-wash trays).
• Acid gas cooler (reflux water recovery).
• Acid gas compressor for acid gas injection.
• Reboiler (hot-oil heated).
• Lean amine surge tank.
• Lean amine cooler.
Fig. 2 shows a simplified process flow diagram for the nearly identical J1 and J2 processing trains.
Validation testing
Following recent advances in hybrid-solvent process modeling,1 Spectra and Dow undertook a comprehensive campaign at Jedney to collect plant operating data and gas samples as part of an effort to further validate model development for ongoing improvement of plant process design.
In April 2015, process data and gas samples were taken from both trains under three controlled operating scenarios.
To attain a complete and useful data set, plant conditions were specified to ensure some detectable sulfur remained in the treated gas. Flash gas absorbers were not in operation during the collection period so that recycled flash gas was left untreated. The total sulfur concentration of treated gas, however, is typically lower during normal plant operation.
The three operating cases for the data-collection campaign were:
• Case A, baseline operation, in which gas feed was evenly split between J1 and J2.
• Case B, high-flow operation, in which gas feed was increased to the indicated train.
• Case C, CO2-slip operation, in which the specified train receives the same gas feed as in Case B but uses 25 absorber trays instead of the 30 used in Case B.
Table 1 summarizes key process parameter targets for the two trains' three operating scenarios (designated as J1A-C and J2A-C, respectively) during the April 2015 data campaign. Gas feed into the J1 train contained 0.83-1.86 mol % H2S and 0.98-2.03 mol % CO2, with feed gas into the J2 train containing 1.13-1.76 mol % H2S and 1.83-2.45 mol % CO2.
Data collection
Comprehensive processing records collected from the gas plant's central data acquisition system provided the core data set. Complementary data including temperatures, pressures, and flow rates also were taken from field instrumentation, including installed gauges and infrared temperature guns.
In addition to samples of flash gas, acid gas, and sales gas (after the refrigeration plant), the data campaign included gas samples from the two trains' absorber inlets and outlets. These samples were collected into Tedlar bags and analyzed for trace sulfurs within two days of collection. Stainless-steel gas sampling cylinders provided samples for routine hydrocarbon analysis (C1-C15+).
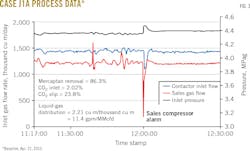
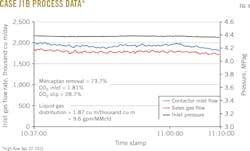
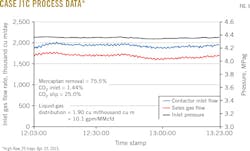
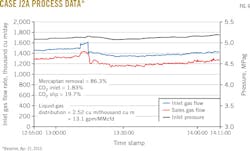
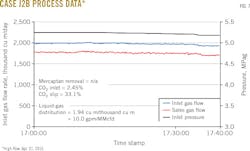
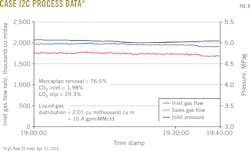
Figs. 3-8 show snapshots of real-time operating data recorded in April 2015 for each of the two trains' three operating scenarios. Table 2 summarizes operating data collected from both trains during the 2015 campaign.
Combined with operating records obtained in earlier collection campaigns at Jedney, data collected in April 2015 provided a general overview of the hybrid solvent's effectiveness in removing acid gas and mercaptans from sour-gas feeds.
Table 3 shows an historical analysis of selected treated and sales gas data from Jedney's two processing trains, including data collected in April 2015 and during previous collection events in 2013 and 1998.
Results
After April 2015's testing and data-collection campaign characterizing process performance of the Jedney plants two trains:
• Mercaptan removal when using 30 trays averaged 82%.
• Reducing the active tray count to 25 from 30 lowered average mercaptan removal to 76%.
• CO2 slip with 30 trays averaged 26.3%.
• CO2 slip with 25 trays nominally improved to 27.2%.
• Solvent-to-gas ratios above 2 cu m/thousand cu m averaged 21.8% CO2 slip and 86.3% mercaptan removal.
• Cases with less than 2 cu m/thousand cu m solvent-to-gas ratios averaged 30.9% CO2 slip and 73.7% mercaptan removal.
Results showed a tradeoff between mercaptan removal and CO2 slip, with higher circulation providing increased mercaptan removal and less CO2 slip.
At the Jedney plant, which has an acid gas injection well, higher CO2 slip reduces the volume of acid gas requiring compression. In plants with a Claus sulfur recovery unit, CO2 slip also can be important, as a higher H2S-to-CO2 ratio in the acid gas is preferable.
Solvent water concentration
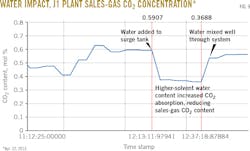
An additional test examined the effect of solvent water concentration on gas-treating performance to determine if water concentration affects the solvent's ability to absorb CO2.
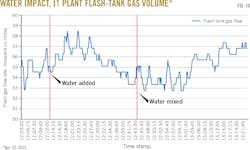
After run J1C, water was added to the lean-solvent surge tank, increasing surge vessel contents to 27.7 wt % from 24.6 wt % water (a relative increase of about 13%). A concurrent change in the sales-gas composition showed a decrease in CO2 to 0.38% from 0.59%. This later rose to 0.56% as the charge of water to the surge tank became well mixed with the full solvent inventory (Fig. 9).
The water-content change also affected flash-gas volume, the increasing water concentration lowering hydrocarbon solubility. An observed dip in flash gas (Fig. 10) indicated that higher water content in the solvent reduces coabsorption of hydrocarbons contributing to flash gas volumes.
These sensitivity data suggested a need to manage solvent-water concentration in real-world operations.
Process simulation
Following the 2015 data-collection campaign, Spectra and Dow performed simulation studies for purposes of cross-validating operating data and gas sampling results and evaluating both the new simulation model's ability to predict H2S, CO2, and mercaptan removal, and its accuracy in predicting acid gas volumes.
Simulation work used ASPEN software and property sets specific to Dow's line of hybrid solvents.1
Table 4 summarizes comparisons of simulated vs. actual plant data.
References
1. Ortiz-Vega, D., Dowdle, J.R., Cristancho, D., Badhwar, A., and Lambrichts, J., "Modelling Acid Gas and Mercaptan Removal with Hybrid Solvents," Sour Oil & Gas Advanced Technology, Abu Dhabi, Mar. 22-26, 2015.
2. Judd, B., and Sterner, T., "A New Hybrid Solvent Application at the Westcoast Gas Services Inc. Jedney Gas Plant," 48th Laurance Reid Gas Conditioning Conference, Norman, Okla., Mar. 1-4, 1998.
The authors