It's possible to derive TBP from partial distillation data
Dicho Stratiev
Angel Nedelchev
Rosen Dinkov
Lukoil Neftochim Bulgaria
Bourgas, Bulgaria
Assen Batchvarov
University of Edinburgh
Edinburgh, Scotland
Distribution characteristics of crude oils and crude oil fractions, as measured by ASTM D-2892, D-5236, D-86, D-2887, and D-1160, can be accurately described by Riazi's boiling point distribution model.1
A full-range distillation curve can then be generated from an incomplete distillation data set. Specifically, the full-range crude true boiling point (TBP) curve can be generated from only the atmospheric part of the crude distillation analysis.
A further improvement in the speed of generating the full-range crude TBP curve can be achieved by converting the ASTM D-86 distillation data of the crude fraction boiling up to 300° C. into TBP and applying Riazi's boiling point distribution model.2 With this approach, the crude oil curve TBP can be generated in 45 min instead of the 48 hr typically necessary for crude oil TBP analysis.
The method proposed in this article can be used in refineries that frequently run on different crudes for generating the full crude oil TBP curve from the simple and quick ASTM D-86 analysis of the distillation characteristics of the crude fraction boiling up to 300º C.
Five different crude oils originating from Russia, Kazakhstan, Azerbaijan, and Iraq were analyzed for their distillation characteristics in accordance with ASTM D-86, D-2892, and D-5236. Naphtha, kerosine, diesel, and vacuum gas oil fractions obtained from these crude oils by fractionation on a TBP apparatus were analyzed for their distillation characteristics in accordance with ASTM D-86, D-2887, and D-1160.
It was found that both crude oil and derived distillate fractions obey Riazi's boiling point distribution model, regardless of the method employed for measuring distillation characteristics.
Based on this finding and a correlation developed in Lukoil Neftochim Bourgas, Bulgaria, that converts ASTM D-86 into the TBP of the crude oil fraction boiling up to 300º C., a method is here proposed to generate the full TBP curve (from 0 to 99%) from ASTM D-86 distillation data.
Changing crude slates
In the current economic climate, refineries face the heavy task of improving the efficiency of individual units, while diversifying product ranges. Processing so called "opportunity crudes" is one option for improving refinery economics.3 Diversifying the crude slate is also a feasible way to improve refinery profitability.4
The frequent change of the refinery crude oil, however, is a major challenge for process engineers who must always determine the optimal operating conditions for the new feed.5-7 In order to cope with these problems, process engineers should have access to crude oil laboratory analyses. The lack of crude oil distillation data may result in wide swings in product qualities.
The single and most important laboratory analysis is true boiling point distillation, which tends to separate individual mixtures sharply and thus give a very good approximation of the expected separation.
Obtaining TBP data is costly and time consuming, however, requiring use of expensive laboratory equipment. A single TBP distillation can take up to 48 hr, which makes it impractical for daily monitoring of refinery crude quality. The Engler distillation (ASTM D-86) is a fast and low cost method for measuring distillation characteristics of oils. It is performed at atmospheric pressure, and for that reason the maximum temperature at which the distillation is terminated is about 360º C. When the Engler distillation is used to analyze crude oil samples, the recommended final boiling point of the distillate is not higher than 300º C.8
A few correlations have been developed that convert ASTM D-86 into TBP distillation data for oil fractions, but one that converts crude oil ASTM D-86 into TBP distillation data has not been developed. Even if such a correlation were developed, it would still be able to give accurate information for the TBP data up to maximum 360º C.
Thus one needs knowledge of a boiling-point distribution model that would be able to describe the full distillation curve for crude oil. Kaes has proposed the use of distillation probability paper to obtain the full distillation curve (from 0.1 to 99.9% evaporate).9 Riazi has proposed a continuous model for C7+ fraction characterization of petroleum fluids.1 Using these two approaches can yield the entire distillation range (from 0 to 99%) from an incomplete distillation data set.
This article investigates the validity of Riazi's boiling point distribution model on distillation data of five different crude oils and derived distillation fractions. The aim is to rationalize the possibility of developing a correlation that converts crude ASTM D-86 into TBP distillation data and, based on this correlation, to propose a method that is capable of generating the full-range crude TBP curve from ASTM D-86 data.
Five crudes
The crude samples analyzed in this work are of various origins:
• Russian export blend crude oil (REBCO); Russia.
• Siberian light crude oil (SLCO); Russia.
• Kirkuk; Iraq.
• CPC; Kazakhstan.
• Oil blend; Azerbaijan.
TBP distillation of all five crudes was carried out in ROFA Deutschland GMBH's EuroDist System TBP column according to ASTM D-2892 (initial boiling point; IBP: 360º C.). The vacuum analysis (above 360º C.) according to ASTM-D 5236 was done with an EDS pot still. The TBP distillation was performed in the EDS TBP column at pressure drops ranging from 760 mmHg to 2 mmHg and in the EDS pot still column from 1 to 0.2 mmHg.
Distillation of all the oil fractions was simulated according to ASTM D-2887. The gas oil fractions were also distilled according to ASTM D-1160. The distillation characteristics for both crude oil and oil fractions were also obtained with the ASTM D-86 procedure. Density at 20º C. was analyzed according to ASTM-D 1298.
According to Riazi, Equation 1 (shown in the accompanying equations box) predicts the distribution of the boiling curve. The suggested property distribution model can be used for physical and chemical characterization of each 1% narrow cut of crude oil.2
With Equation 2 (the linear form of the Equation 1), the parameters C1 and C2 can be determined from the slope and intersect of the graph. T0 is in fact the IBP (T at x = 0) but has to be determined from actual data with x > 0.
It should be noted here that T0 does not always coincide with the measured IBP and has been determined as the lowest positive value at which the highest squared correlation coefficient (r2) for the investigated data set is obtained. This approach is reasonable because the IBP is measured with the lowest accuracy.
Several works on developing empirical methods for converting ASTM distillations into TBP appeared in 1950s and 1960s.10-16 In the mid-1980s, Riazi and Daubert developed analytical methods for converting distillation curves based on the generalized correlation shown in Equation 3.17
For oil fractions, Riazi and Daubert found that constant (c) for all points was zero; therefore Equation 3 reduces to Equation 4.
Crude comparisons
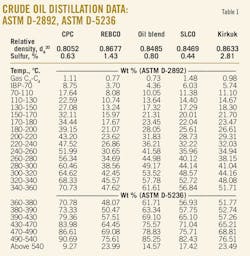
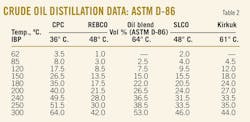
Tables 1 and 2 present distillation characteristics of the five investigated crude oils measured by ASTM D-2892, D-5236, and D-86. The data show, according to the combination of ASTM D-2892 and D-5236 and also according to ASTM D-86, that the crude oil residue content decreases in the following order: REBCO > Kirkuk > SLCO > Oil blend > CPC.
If we compare the relative densities of these crude oils, we can see that they decrease in the order: REBCO > Kirkuk > Oil blend > SLCO > CPC. The order of decreasing densities is almost the same as that of decreasing crude oil residue content, except for the crude oil blend that has lower content of residual fraction than the SLCO but its density is higher than that of SLCO. Therefore the density of a crude oil may not always indicate the content of the high-value distillate fractions.
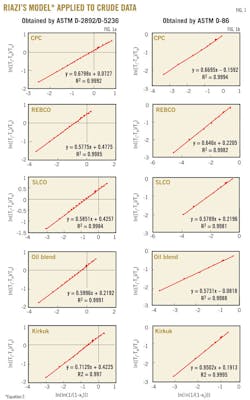
Fig. 1 illustrates the application of Riazi's boiling point distribution model (Equation 2) to the crude distillation data obtained by the combination of ASTM D-2892 and D-5236, and the ASTM D-86. These data indicate that the boiling point distribution model of Riazi fits very well to the measured distillation characteristics of the five crudes. The squared correlation coefficient r2 is higher than 0.99.
These data lead to the conclusion that extrapolation to the crude final boiling point (99%) could be safely made by Riazi's boiling point distribution model because the (r2) of the linearized model is very high and there is no reason divert from the straight line for a distillate content ranging between 0 and 91%.
Table 3 presents data of the values of the parameters AT, BT, and T0 of Riazi's boiling point distribution model calculated from the crude distillation characteristics. The T0 was estimated as the value at which the squared correlation coefficient was maximized. The parameter BT for this data set varied in the range 1.29-1.71. As shown earlier,18 the parameter BT is not a constant value of 1.5 as Riazi reported.1
The ASTM D-86 data show that there is a difference between the measured IBP and the estimated T0. The difference is not significant and could be explained by the fact that the IBP is measured with the highest inaccuracy among other boiling points.2
Gap; overlap
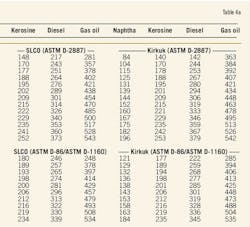
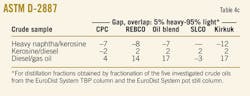
Table 4a presents data of the distillation characteristics of oil fractions derived from the investigated crude samples (heavy naphtha, kerosine, diesel, and vacuum gas oil) measured by ASTM D-2887, D-86, and D-1160. These data give information about the efficiency of the separation in the EDS's TBP column and EDS's pot still column during fractionation of the investigated crudes.
The ASTM D-2887 (simulation distillation) is equivalent to the TBP. Therefore the ASTM D-2887 distillation characteristics of the oil fractions can be used to evaluate the TBP apparatus separation efficiency. Fractionation efficiency is often stated in terms of "gap" or "overlap" between two adjacent side products.
The "gap" and "overlap" concept is based on the difference of the ASTM D-86 5% boiling point of the heavier product and the 95% point of the lighter product.19 When this difference is positive, there is a "gap" indicating good fractionation. A negative difference, called an "overlap," indicates that some of the light products are present in the heavier product and vice versa.20
Table 4b presents the calculated "gap" for the oil fractions, which were characterized by ASTM D-86 and D-1160. The results indicate an effective and reliable fractionation in the context of the described concept; however, they also demonstrate deviation in the gap for each pair of oil products derived from a particular crude oil sample.
The temperature difference between 5% of the kerosine fraction and 95% of the heavy naphtha varied 16° C. to 22° C. The gap for the kerosine and diesel fraction varied in a more narrow range: 29° C. to 31° C. The widest variation in the gap appears for the pair diesel-gas oil: 33° C. to 54° C. The difference in the gap suggests different efficiencies of separation for the oil fractions during the fractionation of crude oils that have different distillation characteristics.
Two major observations applied:
1. The heavier adjacent fractions have a higher gap, which corresponds to higher fractionation efficiency.
2. For both heavy naphtha-kerosine and diesel-gas oil pairs, there are samples that deviate from the pair's trend. For heavy naphtha-kerosine, Kirkuk has an average gap of 16° C., whereas for the other samples the average value is 21° C. The diesel-gas oil pair for CPC has an average gap of 33° C. and for SLCO –44° C., whereas the observed average value for the specified pair is 52° C.
Similar observations were made applying the "gap" and "overlap" concept to distillation data, derived according to the ASTM D-2887 standard (Table 4c). The major difference is that an overlap was observed for the pair heavy naphtha-kerosine. A negative temperature difference exists in the range of –12° C. to –7° C. Its deviation, however, follows the same trend as that of the samples, characterized according to ASTM D-86 standard, which indicates the significance of the methods employed.
The overlap between heavy naphtha-kerosine products and the minimum gap between kerosine-diesel (average 2° C. for all samples) and diesel-gas oil (average 10° C. for all samples) should be related to the specific issues of ASTM D-2892 and ASTM D-2887, which are considered as true boiling point distillation.
Distribution efficiency
There are two issues that have to be taken into account during interpretation of the results derived from TBP distillation. TBP analysis equipment is designed for 15 theoretical trays and a reflux ratio of 5:1. The efficiency of the particular distillation equipment is based on a standard procedure for calculation of the number of theoretical plates. It must be between 14 and 18 in order to meet the specifications stated in ASTM D-2892. There are no clear criteria, however, which allow the assessment of the distillation efficiency. The same statement is valid for ASTM D-2887.
The continuous nature of the petroleum mixtures presupposes an uncertainty in interpretation of the TBP distillation data. It may be concluded that the efficiency of the fractionation of the side products contained in the crude oil at fixed standard conditions (ASTM D-2892, ASTM D-5236, ASTM D-2887, ASTM D-86, and D-1160) depends on the specific boiling range distribution of the crude oil sample.
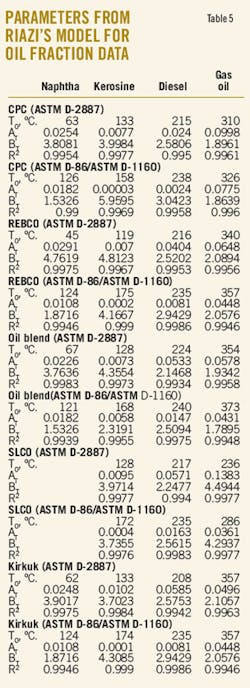
Table 5 presents the values of the parameters AT, BT, and T0 computed from the distillation characteristics of oil fractions by application of the boiling point distribution model of Riazi. The data indicate that, as for the crude oils, the derived oil fractions also obey Riazi's boiling point distribution model. The squared correlation coefficient (r2) of Riazi's linearized model is higher than 0.99.
Once again the calculated T0 is different from the measured IBP. The explanation could be found in the fact that the IBP is measured with highest inaccuracy. It can be seen from these data that both crude oils and the oil fractions derived from them obey the same boiling point distribution model. From an incomplete distillation data, set the full distillation curve (from 0 to 99%) could be generated by estimating the parameters AT, BT, and T0 from the available distillation data.
That correlations have been developed to convert ASTM D-86 into TBP for oil fractions and that crude oil and crude oil fractions obey the same boiling point distribution model may suggest that a correlation that converts crude ASTM D-86 distillation data into TBP should also exist. In previous work,21 Equation 4 with the constants a and b for oil fractions derived by Riazi2 was tested with ASTM D-2892 and D-86 data of 33 crude oil samples. The absolute average deviation for this data set was found to be 19° C. for the crude oil fraction boiling up to 50 wt %.
Application of the Daubert correlation22 to the same data set gave an absolute average deviation of 22.3º C. for the crude oil fraction boiling up to 50 wt %. Application of the Edmister conversion method13 gave an absolute average deviation of 44.3º C. for the crude oil fraction boiling up to 50 wt % .
Application of the correlations developed for conversion of ASTM D-86 into TBP of oil fractions for converting crude oil ASTM D-86 distillation data into TBP turned out to be inconclusive. Therefore, an analyses on 119 different crude oil samples, according to ASTM D-2892 and ASTM D-86 distillation, was carried out at the Lukoil Neftochim Bourgas refinery.
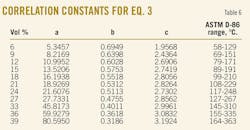
The crude samples have relative density (d420) varying in the range between 0.792 and 0.911. It was found that the generalized correlation (Equation 3) proposed by Riazi and Daubert gave an absolute average deviation of 5.7° C. for 119 crude samples and 1,190 data points.23 Table 6 gives the constants a, b, and c for Equation 3. These constants were derived by processing the data for the 119 crude oil samples, where the temperatures for that case are in Celsius and the specific gravity of Equation 3 was replaced for that particular case by the relative density (d420).
The ASTM D-86 distillation data are in vol %, while those of TBP is in wt %. This means that the constants a and b from Table 6 can be used to convert ASTM D-86 crude distillation data into TBP when the temperatures are in Celsius and the constant c is related to crude relative density d420.
TBP procedure
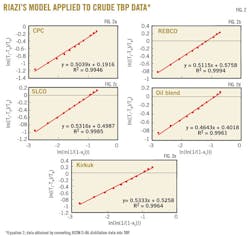
The results show that Equation 3 with the constants a, b, and c can be used to convert ASTM D-86 into TBP for the distillation range between 6% and 39%. Applying Riazi's distribution model to the predicted TBP boiling points of the distillation between 6 and 39% allows the parameters AT and BT to be estimated. For T0 the boiling point of isobutane T = –11.7° C. can be used.1 Fig. 2 is an illustration of the very good agreement between what is predicted by Equation 3 with the constants a, b, and c from Table 6 TBP boiling points and Riazi's boiling point distribution model.
The squared correlation coefficient r2 is higher than 0.99. What is calculated from these data parameters AT, BT, and T0 = –11.7º C. can be used to generate the full-range TBP curve from the ASTM D-86 distillation data of the crude oil fraction boiling up to 300º C. Generating the full TBP curve (from 0 to 99%) from the ASTM D-86 distillation data for the five investigated crude samples requires the following procedure:
1. Determination of Riazi's boiling point distribution model parameters (AT, BT, and T0) from the ASTM D-86 distillation data given in Table 1 (see Table 3).
2. Generation of the full-range ASTM D-86 distillation curve by using Equation 1 and the parameters AT, BT, and T0 obtained in step No. 1.
3. Conversion of the ASTM D-86 distillation vol % (6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, and 39 vol %) into TBP wt % by applying Equation 3 and using the constants a, b, and c from Table 6.
4. Determination of Riazi's boiling point distribution model parameters AT, BT, and T0 for the predicted TBP data by using Equation 2 and the TBP wt % generated in step No. 3 (see Fig. 2). T0 = –11.7º C.
5. Generation of the full-range TBP distillation curve by using Equation 1 and the parameters AT, BT, and T0 obtained in step No. 4.
Table 7 gives a comparison between measured distillation characteristics (content of the fractions: naphtha, kerosine, diesel, and vacuum gas oil, and TBP temperature at several wt % distilled) of the five investigated crude oils by the combination of ASTM D-2892 and D-5236 and what is predicted by the method described above based on ASTM D-86 data.
Table 7 also presents data of vacuum gas oil and vacuum residue content in the five crude oils estimated from the ASTM D-2892 distillation data by incorporating Riazi's boiling point distribution model. The data show that application of the boiling point distribution model of Riazi to the atmospheric part of the EDS TBP column (ASTM D-2892) allows prediction of the vacuum gas oil (VGO) and vacuum residue (VR) content without performing ASTM D-5236 vacuum pot still analysis.
In that case the full-range TBP curve could be generated for 12 hr instead of 48 hr by performing only ASTM D-2892 analysis. The accuracy of prediction of VGO and VR content for all investigated five crude oils with the exception of Kirkuk crude is within the reproducibility of ASTM D-5236. If the T0 in Riazi's boiling point distribution model is set to be –11.7º C. (boiling point of isobutane as Riazi has proposed1), however, the accuracy for predicting VGO and VR content is considerably improved. (See data in parentheses in Table 7.)
Therefore, an accurate full-range crude TBP curve could be generated only from the atmospheric part of the EDS TBP column (ASTM D-2892) and use of Riazi's boiling point distribution model by assuming T0 = –11.7º C.
If we look at the prediction of the full-range TBP curve from the ASTM D-86 distillation data, we can see an excellent agreement for REBCO, a very good agreement for SLCO, an adequate agreement for the oil blend and Kirkuk crudes, and not very good agreement for the CPC crude. The generation of the full-range TBP curve from the ASTM D-86 distillation data can be performed within 45 min; therefore, this method could be used for daily monitoring of crude quality in a refinery.
Improvement in the prediction of TBP up to 300° C. from the ASTM D-86 distillation data of the crude fraction boiling up to 300° C. may improve considerably the full-range crude TBP curve prediction. The next task for the LNB research laboratory is to search for approaches to improve the conversion of crude ASTM D-86 into TBP.
Riazi's boiling point distribution model proved its ability to generate the full-range distillation curve from an incomplete distillation data set.
References
1. Riazi, M., "A Continuous Model for C7+ Fraction Characterization of Petroleum Fluids," Ind. Eng. Chem. Res., 36 (1997), No. 10, pp. 4,299-4,307.
2. Riazi, M.R., Characterization and Properties of Petroleum Fractions. Philadelphia: American Society for Testing and Materials, 2005.
3. Ooms, A.C., Van den Berg, F., Kapusta, S.D., and Nouwens, L.W., "Processing opportunity crudes: A new strategy for crude selection," Proceedings, ERTC 2001. Madrid.
4. Stratiev, D., Dinkov, R., Nikolaev, N., and Stanulov, K., "Evaluation of impact of crude oil quality on refinery profit," Erdoel Erdgas Kohle, 126 (2010), No. 1, p. 17.
5. Ebbesen, L.E., "Main fractionator crude switch control," Computers & Chemical Engineering, Vol. 16 (1992), No. 1, pp. s165-s17.
6. Dave, D.J., Dabhiya, M.Z., Satyadev, S.V.K., Ganguly, S., and Saraf, D.N., "Online tuning of a steady state crude distillation unit model for real time applications," Journal of Process Control, Vol. 13 (2003), No. 3, pp. 267-82.
7. Kumar, V., Sharma, A., Chowdhury, I.R., Ganguly, S., and Saraf, D.N., "A crude distillation unit model suitable for online application," Fuel Processing Technology, Vol. 73 (2001), No. 3, pp. 1-21.
8. Gosudarstvennye Standarty State Standard (GOST) 2177: Petroleum products. Methods for determination distillation characteristics; Sept. 21, 1999.
9. Kaes, G.L., Modeling of Oil Refining Processes and Some Practical Aspects of Modeling Crude Oil Distillation, a part of VMGSim User's Manual, www.virtualmaterials.com.
10. Nelson, W. L., and Hansburg, M., "Relation of ASTM and True Boiling Point Distillations," Oil & Gas Journal, Aug. 3, 1939, p. 45.
11. Edmister, W.C., and Pllock, D.H., "Phase Relations for Petroleum Fractions," Chemical Engineering Progress, Vol. 44 (1948), pp. 905-26.
12. Edmister, W.C., and Okamoto, K.K., "Applied Hydrocarbon Thermodynamics, Part 12: Equilibrium Flash Vaporization Correlations for Petroleum Fractions," Petroleum Refiner, Vol. 38 (1959), No. 8, pp. 117-32.
13. Edmister, W.C., and Okamoto, K.K., Applied Hydrocarbon Thermodynamic. 2nd ed. Houston: Gulf Publishing, 1961, pp. 116-33.
14. Edmister, W.C., and Okamoto, K.K., "Applied Hydrocarbon Thermodynamics, Part 13: Equilibrium Flash Vaporization Correlations for Heavy Oils Under Subatmospheric Pressures," Petroleum Refiner, Vol. 38 (1959), No. 9, pp. 271-88.
15. Okamoto, K.K., and van Winkle, M., "Equilibrium Flash Vaporization of Petroleum Fractions," Industrial and Engineering Chemistry, Vol. 45 (1953), pp. 429-39.
16. House, H.G., Braun, W.G., Thompson, W.T., and Fenske, M.R., "Documentation of the Basis for Selection of the Contents of Chapter 3, ASTM, TBP, and EFV Relationships for Petroleum Fractions in Technical Data Book—Petroleum Refining," Documentation Report No. 3-66, Pennsylvania State University, 1966.
17. Riazi, M.R., and Daubert, T.E., "Analytical correlations interconvert distillation curve types," Oil & Gas Journal, Aug. 25, 1986, p. 50.
18. Stratiev, D., Dinkov, R., Kirilov, K., and Petkov, K., "Method calculates crude properties," Oil & Gas Journal, Jan. 7, 2008, p. 48.
19. Watkins, R.N., Petroleum Refinery Distillation. 2nd edition. Houston: Gulf Publishing Co, 1979.
21. Jones, D.S., and Pujado, P.R., Handbook of Petroleum Processing. Dordrecht: Springer, 2006.
21. Nedelchev, A., Stratiev, D., Ivanov, A., and Stoilov, G., "Boiling point distribution of crude oils based on TBP and ASTM D-86 distillation data," submitted to Petroleum and Coal.
22. Daubert, T.E., "Petroleum Fraction Distillation Interconversion," Hydrocarbon Processing, Vol. 73 (1994), No. 9, pp. 75-78.
23. Lukoil Neftochim Bourgas. Internal Report: May 2011, Bourgas: Lukoil Neftochim Bourgas.
The authorsMore Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com