PETCOKE & METHANOL—1: Flow scheme envisions producing methanol for gasoline blending
David Netzer
Consulting Chemical Engineer
Houston
Chris Wallsgrove
Brinderson Engineers & Constructors
Costa Mesa, Calif.
The bitumen upgraders in the Fort McMurray area of Alberta and the refineries in the Edmonton area are large producers of petroleum coke (petcoke), which, historically, has had essentially zero market value in Alberta.
Petcoke, however, can be converted to methanol, which can be used as a gasoline blending component or marketed as chemical-grade material. The conventional method of producing methanol, outside of China, is by steam reforming of natural gas.
This two-part series analyzes the economics of producing 50,000 b/d of methanol for gasoline blending from petcoke and examines some aspects of using methanol-gasoline blends for motor fuel. Corn-based ethanol-gasoline (10 vol % blend) serves as a reference case.
Part 1 presents the production flow scheme we have developed and discusses utilities requirements and basic process parameters. The concluding article (OGJ, July 4, 2011) will present the issues associated with using methanol blended with gasoline as a fuel for cars and light trucks.
Producing syngas
Production of synthesis gas (including that for methanol production) by partial oxidation of solid fuels, including petroleum coke, is not a new concept but is not very frequently practiced. The great majority of methanol plants around the world, outside of China, are based on steam-methane reforming of natural gas. They are often located where natural gas is priced very competitively. By contrast methanol production in North America and Europe has been declining due to poor economics.
The use of methanol as a precursor for performance chemicals such as formaldehyde or acetic acid and derivatives, although significant, nevertheless represents a limited market compared to the potential of using methanol as a gasoline blend. Also significant is the potential use of methanol to make dimethyl ether (DME), which could be used as a substitute for arctic diesel or as an LPG blending component. Conversion of methanol to olefins is also feasible, but lies outside the scope of this study.
The idea of using methanol as a gasoline blend, especially at high concentrations such as M-85 or even pure methanol (M-100), has been around and tested in the US for decades and is currently being practiced in several provinces in China. Nevertheless, the methanol option has faced strong political objections, principally based on its assumed toxicity or on motor vehicle modification issues.
Particularly fierce is competition with ethanol, which has been considered a “renewable fuel” (22% renewable by the US Environmental Protection Agency).1 The present analysis considers methanol at concentrations of 7-10 vol % in gasoline, equating the fuel oxygen content to current ethanol-gasoline blends.
Recent studies by others for converting coke to methanol were driven by the desire to maximize thermal efficiency and to reduce CO2 release to a bare minimum.2 These studies contemplated about 95% CO2 capture with an integrated gasification combined cycle (IGCC) hydrogen power cycle. Unfortunately, this approach resulted in much higher capital investments compared with the proposed concept, which proposes to capture only about 67% of the CO2, comparable to using ethanol as a blend component in gasoline. None of these studies has materialized into actual projects.
The objective of the present discussion is to re-examine objections to methanol as a gasoline blending component in North America. We present an objective analysis of the assumed toxicity issue(s) and of the renewable fuel issue(s). We compare the release of CO2 from production and combustion of methanol with the CO2 released from the production and combustion of corn-based ethanol.
The current EPA-mandated 10 vol % ethanol-gasoline blend serves as a reference case for economic, environmental, and vehicle-performance analysis. Note that Canada currently mandates a 5% ethanol-gasoline blend.3
The capital investment required to produce methanol from petcoke is considerably higher than from natural gas (probably about 2.5 times), depending on the CO2-capture system.4 Nevertheless, in northern Alberta where petcoke has an essentially zero market value, there exists a unique synergism for the competitive production of methanol.
This tipping point could occur when the price of natural gas exceeds about US$3.00-5.50/MMbtu (low heating value), depending upon the possible opportunity to use CO2 for enhanced oil recovery. Nevertheless, for crude oil West Texas Intermediate (WTI) at more than $75/bbl, the historic price ratio of natural gas to crude oil suggests a “normal” natural gas price of about $8/MMbtu.
The total conversion efficiency, in our conceptual flow scheme, of coke plus coal energy to methanol and hydrogen (excluding the energy in the sulfur and power export) is about 52.5% on high heating value. This was calculated by summing the energy content of the products and dividing by the sum of the energy content of the feedstocks. This compares with perhaps 44 to 47% on HHV using coal-to-liquids via Fischer-Tropsch (FT) synthesis.5
The model of methanol production for gasoline blending was selected to be 50,000 b/d (6,400 tonnes/day; tpd) from two synthesis reactor trains. This rate represents close to the maximum capacity for a commercially sized unit. Slurry coke gasification at 87 bar has been selected to gasify a petcoke-water slurry at 1,425° C. Unconverted carbon is used as boiler fuel, along with supplementary coke and/or coal fuel, to generate high-pressure steam to power the plant.
The conceptual estimated capital investment in the Edmonton area, assuming “overnight” construction, is around US$2.8 billion, depending upon CO2 capture requirements. With $25/tonne (dry basis) cost of coke ($0.75/MMbtu HHV) and $1.50/MMbtu HHV (as received) coal, the production cost of methanol is about $0.45 to $0.88/gal ($150-292/tonne). This depends upon on the possible CO2 capture and sales for enhanced oil recovery (EOR) and upon the expected payback or rate of return on capital. The high political stability in Canada and the maturity of the technology would tend to a support lower rate of return on the investment.
On a volumetric energy basis, this would be equivalent to $0.92-1.79/gal of gasoline before tax and distribution costs (October 2010 basis), albeit with higher octane and lower vehicle emission. On the US Gulf Coast, corn-based ethanol as gasoline blend stock is sold to distribution (October 2010 averages) for about $1.50/gal after $0.45 tax rebate, equal to a total of $1.95/gal before tax rebate. After correction for volumetric energy content, this is equivalent to about $3/gal of regular gasoline before tax and distribution costs.
Petroleum coke feed
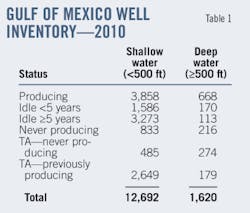
In this particular configuration, 7,400 tpd (dry basis) of petcoke from delayed-coking sources (such as Suncor) enters the gasification plant section. Our material balance assumes the coke composition (on a dry basis) shown in Table 1.
The incoming coke undergoes wet grinding to a fine mesh and forms a coke slurry consisting of 61 wt % coke (as dry), 1% fluxing agent such as ash, and 38 wt % water. Use of some 85% coke and 15% subbituminous coal (available in the Edmonton area) could be a viable feedstock alternative, and under this scenario no fluxing agent will be required. The coke slurry proceeds to well mixed and well vented slurry storage tanks.
About 6,600 tpd of oxygen of 99.5 mole % purity (the balance is mostly Argon) and at 100 bar is produced in two identical air separation unit (ASU) trains of 3,300 tpd each. The ASU steps are air compression, drying, CO2 removal by molecular sieve, expansion, and oxygen/nitrogen fractionation in double cryogenic columns.
The selection of the air compression method is governed by start-up consideration, especially in this case when compression power on the order of 63,000 kw/compressor is being considered. Selection could be the subject of a rigorous analysis, based on location, existing infrastructure, power distribution, power purchase contract criteria, and operational preference of the methanol producer(s).
Liquid oxygen pumping, along with variable-speed centrifugal air compressors using 125 bar/500° C. steam turbines, would represent a safe and conservative approach, and this combination was selected for this study. The balance of the complex, including the liquid oxygen pumps, is all driven by electric motors.
Three operating slurry gasifiers, plus one spare, are assumed for the model of the gasification island. The proven ability of slurry gasification technology to operate at 87 bar, thus avoiding downstream compression to methanol synthesis, drives this selection. Note that dry-feed gasification technologies are currently limited to operating at about 40 bar or less.
Quench-mode gasification, although less thermally efficient compared with high-heat-recovery designs (which can produce steam at 130 bar), can nevertheless produce 25 to 30 bar steam. Therefore, the quench mode with heat recovery to 27-bar steam was judged to be far less capital intensive and of lower maintenance and higher on-stream factor. This is particularly true when the value of coke is of the order of $0.75/MMbtu (US$25/ton, dry).
Coke slurry is pumped by motor-driven pumps to a pressure of 100 bar. The pressurized slurry mixes with pressurized oxygen in the combustion nozzle. The resulting synthesis gas is at about 1,425° C. and about 87 bar. This contains about 10% of unconverted carbon and some fluxing agent. The raw synthesis gas is water quenched to adiabatic saturation at about 255° C. The majority of the unconverted carbon is captured in the quench section and the balance in the downstream soot scrubbers.
The total hot synthesis gas rate is about 35,960 kg-mole/hr (753 tonnes/hr; tph). Table 2 shows the gasifier’s gas yield at the combustion nozzle.
After the quench, the water content of the synthesis gas is increased to 58 mole % from 11.7 mole % and the total gas flow increases to 75,600 kg-mole/hr at 84 bar and 255° C. About 810 tons/day, on a dry basis, of residual unconverted carbon, containing heavy metals, fluxing agent, and sulfur, is rejected as filter cake. This is routed as a partial fuel blend to the power generation unit.
Shift, gas cooling
Two 50% shift and gas cooling trains are assumed. Wet and dust-free gas from the gasification island is partially cooled to 250° C. Partial condensation of water brings the water content to 50 mole % while generating saturated steam at 27 bar. The gas is reheated to about 285° C. and enters a single-stage prereactor. Here, traces of formic acid and hydrogen cyanide are destroyed, along with minimal shift conversion.
The gas stream then splits; about 35% of it bypasses the shift reactor. The balance, about 65%, of the gas enters a single-stage sour shift reactor. About 75% of the CO entering this reactor is shifted to CO2 and hydrogen. The shifted gas is cooled by generating 130-bar saturated steam.
This gas stream is combined with the 35% bypassed gas, thereby controlling the ratio of H2/CO as required by the downstream methanol synthesis. This gas cooling by heat recovery into saturated steam is a significant steam producer for the complex.
Acid-gas removal
Selection of a physical solvent for H2S/COS/CO2 removal is almost mandatory in this case, particularly due to the catalyst sensitivity to sulfur species in the downstream methanol synthesis. Given the fact that methanol is the product, using Rectisol, in which methanol is the solvent, would represent a logical approach. Nevertheless, Selexol solvent could be a suitable alternative.
Two 50% Rectisol absorption trains along with a single 100% associated refrigeration unit and single flashed-gas recycle compressor are assumed.
About 39,500 kg-mole/hr (830 tph) of synthesis gas at 80 bar enters the Rectisol systems, containing (in mole %): CO (20); H2 (46); CO2 (31); H2S (1.5); COS (0.02); and Ar+ CH4+ N2 (1.5).
The cold methanol scrubbing occurs in two stages:
1. H2S/COS and partial CO2 removal in a first stage, maximizing H2S to CO2 ratio in the rich solution.
2. Bulk CO2 removal in a second stage, with minimum bypass to control the CO2 content of the methanol synthesis gas. A concentration of 2.5 mole % residual CO2 (about 6% of the inlet) in this is our initial assumption.
Both streams go through three stages of flash to recover the dissolved CO2. The acid gas containing about 50 mole % H2S (with some COS) and 50 mole % CO2 is than routed to a conventional (Claus) sulfur plant.
As an alternate, depending on the business model, this stream could feed a wet sulfuric acid plant. The CO2-rich solution, essentially free of H2S, is recovered to give 11,700 tpd of nearly pure CO2 product. In the present conceptual model, this CO2 is compressed to about 130 bar and routed to underground injection outside battery limits (OSBL). If the CO2 is vented to the atmosphere, then incineration, probably in the boilers, will perhaps be required to eliminate traces of CO.
High-pressure Rectisol processing increases capacity, and the increased flash pressure reduces the compression power needed to deliver the CO2 to sequestration.
The Rectisol process is a significant consumer of refrigeration power, typically using perhaps –40° C. propylene refrigeration.
Sulfur recovery
H2S-rich gas from the Rectisol unit is washed to remove and recover traces of methanol. It flows to one of two (2 × 100%) conventional sulfur-recovery units (SRUs). We estimate 94% sulfur recovery at end-of-run, which will bring the total sulfur production to about 425 tpd. The balance of the sulfur, about 30 tpd, passes to the tail gas, which consists of H2S, SO2, and sulfur vapor, diluted with nitrogen, CO2, and water vapor. This tail gas is sent to incineration in the utilities’ boilers using duct burners; the ultimate sulfur capture is 99.6%.
The final rejection, along with the sulfur entering with the primary boiler fuel, is as a filter cake of gypsum (CaSO4.2H2O). The sulfur plant produces steam for use within the complex. Note that the feed to the sulfur plants contains no ammonia, unlike most refinery SRUs. Therefore the option to have a single SRU is worth consideration. A single-SRU option would reduce the overall capital investment by about $50 million.
Methanol synthesis
Isothermal methanol synthesis in two 50% reactors, with two fractionation trains and a single common recycle compressor, is assumed for this conceptual model.
The methanol synthesis reactor system represents only about 5-6% of the total capital investment. We choose to be conservative and to allow operation flexibility and enhance availability by having two 50% reactors at small incremental capital cost. Further, construction or delivery of “mega” methanol reactors in land-locked Alberta could present some serious logistical issues.
About 302 tph of gas, with the estimated composition shown in Table 3, enters the methanol synthesis at 78 bar.
Synthesis gas is recycled by a centrifugal recycle compressor, with a 2:1 recycle ratio and a pressure range of 73-78 bar. Nitrogen, argon, and methane are removed as a 15% purge. The purge gas, of about 1,730 kg-mole/hr (24 tph), contains (in mole %) H2 (56.5); CO (25.5); CO2 (3.0); inerts (15.0).
This gas flows to a pressure-swing adsorption (PSA) unit for hydrogen recovery at about 40 bar. Roughly 80% of the hydrogen (about 38 tpd) is recovered (i.e., about 16 MMscfd) and goes “across-the-fence” as a revenue-generating product. PSA purge gas at 0.5 bar, 950 Kg-mole/hr (22 tph), containing 46 mole % CO, 20 mole % H2, and 6 mole % CH4 is compressed to 4.5 bar and proceeds to the fuel-gas system.
Generating steam at 27 bar recovers the heat of the methanol synthesis reaction, and this steam (along with steam from the sulfur plant and preshift gas cooling) is superheated in the power cycle, as discussed later. In the current configuration, most of the PSA purge gas is used as steam superheater fuel.
About 6,400 tpd of methanol (50,000 b/d), 300 tpd of water, and perhaps 20 tpd of other organics (such as ethanol and DME) are produced. The crude methanol proceeds to fractionation in a double-column system. This produces commercial specification methanol (typically 99.95% purity).
A gasoline grade of methanol, perhaps 99.7% methanol and 0.3% organics, could be produced in a single dehydration column. For the current conceptual model, however, we elected to produce commercial or chemical-grade methanol as blend stock, for about 1.5-2% higher capital investment. This allows market opportunities for partial sale to the chemical market, at a higher margin.
Fig. 1 shows an overall production scheme.
Utilities system
The concept is for the plant to be self-sufficient in steam and power. Three steam cycles are contemplated:
1. About 525 tph of 27-bar steam is produced by process waste heat. A fired superheater, firing about 80% of the PSA purge gas, superheats this saturated steam to 340° C. The resultant steam goes to the single 100-Mw turboalternator.
2. About 430 tph of 125 bar, 510° C. steam from boilers is used to drive the two, 63-Mw air compressors in the two ASUs.
3. About 400 tph of 125 bar, 510° C. steam from boilers is used to drive the balance of power generation. The turbine for this has about 190 tph of 3.5 bar extraction steam.
Cycles 2 and 3 consist of three, 55% fluidized bed boilers and one 100-Mw electric power generator. There is no spare air compressor.
The third, spare boiler produces steam for ~80-Mw of power generation; this “surplus” power is available for export. If power is not exported, the power generator operates at very low capacity with some reduction in efficiency. The boiler superheating sections accept the 130-bar saturated steam produced in the shift and gas cooling sections. If one boiler is down, then export power for revenue ceases.
Because of the high metals content, mostly vanadium oxides, in the spent carbon, a partial coal feed is used in the boilers as fluxing agent to enhance fluidization. The metals leave with the spent limestone and gypsum to regulated landfill.
Note that the utilities’ consumptions and balances are based upon summer conditions, with 25° C. ambient temperature. Fig. 2 provides a flow scheme of the steam and energy systems.
Fuels
Six fuel sources feed the utilities system:
1. Unconverted carbon, 810 tpd on a dry basis, from the gasification section.
2. About 20% of the PSA purge gas to the boiler’s duct burners, along with the sulfur plant’s tail gas.
3. About 80% of the PSA purge gas to the medium-pressure steam superheater.
4. A 50/50 wt % mixture of petcoke and subbituminous coal.
5. Vent gases from the sulfur pit and gray water flash vapor from gasification .
6. Waste organics from methanol purification.
The coal feed analysis shows an HHV of 8,600 btu/lb as received with 50.5 wt % carbon.
The boilers are fluidized bed types, using limestone as the bulk-sulfur capture medium. About 90% of the sulfur from the fuel is captured within the fluidized bed. The flue gas, after the superheater, economizer, nitrogen oxide removal, and air preheater sections, is scrubbed with wet limestone. This brings the overall sulfur capture to about 97%, all of which is disposed of as gypsum sludge. Limestone consumption is estimated to be about 550 tpd; the capital cost of the limestone and coal receipt, storage, and handling is included with the boilers.
The accompanying box summarizes the utilities. Note that cooling-water power consumption includes cooling-water pumps and tower fans.
Water consumption
Water consumption, which is mostly evaporative losses from the cooling towers, is on the order of 55,000 tpd. This amounts to about 0.3% of the estimated water flow in the North Saskatchewan River. About 70% of the cooling water duty is attributed to turbine exhaust surface condensers.
Table 4 shows process steam balance.
Boiler-fuel consumption with power export:
1. Spent carbon (11,200 btu/lb HHV), 810 tpd (dry basis).
2. 50/50 wt coal/petcoke (11,175 btu/lb HHV), 1,520 tpd (as received).
3. PSA purge gas (to duct burners), 10 g-cal/hr LHV.
Boiler-fuel consumption without power export:
1. Spent carbon, 810 tpd.
2. 50/50 wt coal/petcoke, 640 tpd.
3. PSA purge gas (to duct burners), 10 g-cal/hr.
Note that the 27-bar steam fired superheater uses about 40 g-cal/hr of compressed PSA purge gas as its fuel.
Acknowledgments
The authors acknowledge the assistance of the following organizations and people: Lurgi GMBH, Frankfurt; Linde Engineering, Munich; HDR Engineering Inc., Ann Arbor, Mich.; Haldor Topsoe Inc., Houston; Dennis Dembicki, Edmonton; John Lehman, Edmonton; Marshal Bell, Los Angeles; and Don Koza, Frederick, Md.
References
The authors1. “Renewable Fuel Standard Program (RFS2) Regulatory Impact Analysis,” US EPA, Report EPA-420-R-10-006, February 2010, Chapter 2.
2. “Economic Assessment of Coal-Based Power Plants with CO2 Capture,” EPRI, Palo Alto, 2008.
3. “Vehicle Fuels,” Office of Energy Efficiency, Natural Resources Canada; oee.nrcan.gc.ca/transportation/alternative-fuels/index.cfm.
4. Budget costs from licensors, plus additional private correspondence.
5. “Baseline Technical and Economic Assessment of a Commercial Scale Fischer-Tropsch Liquid Facility,” US DOE, Report DOE/NETL—2007/1260, April 2007.
In “GUIDE TO WORLD CRUDES: New fields prompt Troll Blend update” (OGJ, Apr. 4, 2011, p. 94), in the footnote to the box “Test conditions: True boiling point distillation,” the conversion should read “Torr. ≈ 1 mmHg ≈ 0.0193 lbf/sq in.”
More Oil & Gas Journal Current Issue Articles
More Oil & Gas Journal Archives Issue Articles
View Oil and Gas Articles on PennEnergy.com