Marathon refineries employ new FCCU CO combustion promoter
To meet objectives of a 2001 consent decree with the US Environmental Protection Agency, Marathon Petroleum Co. LLC, a subsidiary of Marathon Oil Co., agreed to decrease NOx emissions from each of its fluid catalytic cracking units at seven midwestern US refineries.
In satisfying this agreement, Marathon employed a new, second–generation non–platinum combustion promoter, COP–NP, developed by Intercat Inc., Manasquan, NJ. This article describes a series of successful trials of COP–NP in Marathon’s FCCUs and gives relevant side–by–side test results of COP–NP with competitor additives.
Since signing its consent decree, Marathon has worked closely with EPA and other US refiners under similar decrees to ensure the objectives are met reliably and cost–effectively. In keeping with the spirit of these agreements, Marathon has consistently pushed technology boundaries by being among the first to implement many new emission–control technologies.
FCCU and emissions
The FCCU is typically the largest emitter of airborne emissions in a crude oil refinery. The FCCU’s flue–gas stack emissions contain several environmental pollutants, including CO, nitrogen oxides (NOx), sulfur oxides (SOx), and particulates. In the US, beginning in the 1990s with the amendment of the Clean Air Act and subsequent consent–decree agreements, refiners have been required by the EPA to minimize emissions of these compounds.
In Europe, several European Union–wide directives target reductions in emissions of these compounds from the FCC flue–gas stack. In other countries, especially industrialized ones, limits have been placed on flue–gas emissions to minimize these pollutants, which are normally contained in the uncontrolled emission streams.
Several papers detail the terms of the US consent decrees and the European directives.1–4 Other papers have outlined the complex chemistry of the numerous competing reactions that occur in the FCC regenerator and described the technical challenges of minimizing these environmental pollutants while maintaining an economically profitable refining operation.5
One of the stated objectives in Marathon Petroleum’s 2001 consent decree was to decrease NOx emissions from each of its seven FCC units. Catalytic additives were required for NOx reduction and control in five of the FCCUs. The level of control was to be similar to those that could be achieved with hardware modifications, with final limits determined through an 18–month demonstration of performance.
Marathon completed all additive demonstrations and reached agreement with EPA on final limits by yearend 2006.
FCC heat– balance control
The FCC unit operates in heat balance, meaning that the total energy coming into the FCC must equal the energy going out. The primary source of energy in the FCC unit is coke that forms on the catalyst surface. Combustion of coke in the FCC regenerator supplies the energy to run the cracking process.
Total unit heat duty will typically be in the range of 500 to 1,000 btu/lb of feed to the unit. This process heat requirement establishes the amount of heat that must be supplied by combustion of coke. Because of the process control schemes that are normally employed in FCC, the unit operation will automatically adjust itself so that the energy produced via coke combustion equals the heat requirements of the process.
If the balance is shifted by changes to the feed quality or operating conditions, shifts in catalyst circulation rate and regenerator temperature will occur until a new equilibrium set of conditions is established.
The combustion of coke is considered to be a first–order reaction. Operating temperature is a prime variable in setting the coke combustion rates. Since the kinetics are first order, increasing or decreasing the temperature by about 30° F. will double or halve the combustion rate. This can be significant when the coke or air distribution in the regenerator is less than ideal.
The combustion of coke proceeds via carbon oxidation to CO and then CO combustion to CO2. The activation energy for CO combustion is considerably higher than for carbon combustion, so that CO combustion is usually the rate–limiting step in the process. At temperatures less than 1,250° F., the thermally driven reaction rate of CO to CO2 is very slow.
To help speed up the process, CO combustion promoter is added to the unit to increase the CO combustion rate. Because the heat release from CO combustion is about three times greater than the heat release from carbon to CO, it is important that this combustion occur in the dense bed of catalyst.
Without the catalyst bed to absorb this heat of combustion, the flue–gas temperature increases very rapidly—a phenomenon called “afterburning”—and represents an energy loss from the regenerator and a decrease in the overall thermal efficiency of the regenerator.
Use of promoter causes the CO to burn in the catalyst bed so that afterburning is controlled to acceptable levels. The benefits of this control include extending the mechanical life of downstream equipment, lower carbon on regenerated catalyst, improved utilization of air, and decreased catalyst deactivation.
In a typical fluid–bed regenerator, the dense–phase catalyst particles are well mixed by fluidization. Air entering the bottom moves in plug flow up the regenerator. As the combustion reactions progress to remove coke from the catalyst, oxygen is consumed and the combustion products (CO and CO2) change in concentration along the regenerator axis.
Changes in O2 and CO concentration are of particular interest because NO and NO2 are reduced in the presence of CO. The magnitude of change is expected to be different with different regenerator configurations and cocurrent vs. counter–current flow. In addition to regenerator configuration and mode of operation (partial vs. complete combustion), these compositions will also change with the flue CO/CO2 ratio (in partial combustion), excess oxygen (in complete combustion), feed type, and other parameters that affect the coke on the catalyst.
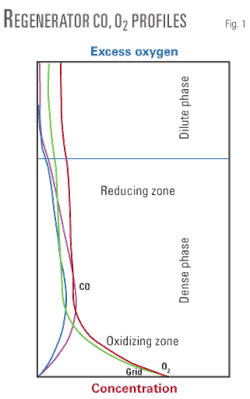
Fig. 1 depicts the expected concentration profiles for CO and O2 with regenerator elevation in a well mixed bed, with data from the Marathon FCC pilot plant.
An increase in excess oxygen would result in less reduction potential in the reducing zone. The relative concentrations of CO and O2 largely dictate the emission chemistry. Thus, the above profile underscores that the emissions cannot be readily assessed from changes in oxygen concentration at the entrance (air grid) or exit (excess O2 in flue) of the regenerator. Rather, it is important to understand the axial variations and their impact on emissions. Industry experience has shown emissions chemistry in particular will be affected by specific unit designs and bed hydrodynamics.
Effects of promoters
The combustion chemistry in the FCC regenerator that produces these pollutants is extremely complex; numerous interactions and reactions occur between the various chemical species. Ultimately, all of the combustion chemistry revolves around the competition for and the availability of oxygen in the regenerator.
It has been shown that catalysts, additives, or process conditions that reduce one undesirable flue–gas component can inadvertently lead to an increase in emissions of another. An especially good example of one of the interactions that occurs in the regenerator is that between CO and NOx.
Laboratory research has shown that even in the oxidizing environment of the regenerator, CO can act as a reductant, reducing NOx to N2. Thus, elimination of CO by complete oxidation of CO to CO2 can result in an increase in NOx emissions from the FCCU.6 It is important to be aware that when attempting to minimize emissions of these undesired chemical species in the flue gas, care must be taken in design and use of different emissions–control technologies. Otherwise unintended consequences and undesirable results could occur.
For nearly 35 years, the preferred method of controlling afterburn and CO emissions has been use of a platinum–containing combustion promoter to catalyze the oxidation of CO to CO2 in the regenerator dense bed. Historically these combustion promoters have contained low concentrations of highly dispersed platinum (Pt) on an inert alumina support.
Pt is an excellent combustion promoter that, unlike many other oxidation catalysts, can be used in low enough concentrations to catalyze CO oxidation without also catalyzing undesirable dehydrogenation reactions in the FCC reactor. It has been discovered, however, that while Pt is an effective oxidation catalyst for conversion of CO to CO2, it also catalyzes the oxidation of nitrogen intermediate species (e.g., ammonia, NH3; and hydrogen cyanide, HCN) found in the regenerator. This results in increased NOx emissions and runs counter to the desire to minimize all of these environmental pollutant emissions.
In recent years, a shift has occurred away from Pt–containing CO combustion promoters to promoters containing elements other than Pt. The desire is to use elements that are still effective in oxidizing CO to CO2 and preventing afterburn, but which do not generate NOx in the process. Many of the currently available non–Pt combustion promoters have substituted palladium (Pd) for Pt as one active component. Commercial performance results, however, show that the performance of these materials varies widely and that additives available from different suppliers are not all equivalent.
Development of COP–NP
In development of a non–Pt CO combustion promoter, initial products substituted a different oxidizing agent for Pt, leaving all other additive properties unchanged. While many of these new additives have been successful in reducing NOx emissions, most are not as effective as CO combustion promoters, requiring from two to five times more additive to achieve the same degree of combustion effectiveness and afterburn control as with a Pt promoter.
Intercat took a different approach in developing its non–Pt combustion promoter, COP–NP. The company completely redesigned the new non–Pt combustion promoter, incorporating modifications to the base support as well as to the catalytically active components. This approach produced a far more active, stable, and selective combustion promoter than others on the market.
In developing COP–NP, Intercat recognized that the effectiveness of a CO promoter in the FCCU depends on several physical and chemical factors. The support must be a highly attrition–resistant material, have a high particle density, and have a minimum amount of particles smaller than 40μ. These properties are necessary to minimize first–cycle losses of the promoter and promote high retention in the unit’s inventory.
The support should also have a moderately high surface area and a high pore volume to provide ready access of the reactants to the catalytically active sites. The catalytically active components must be uniformly dispersed on the support for maximum effectiveness. The chemical composition of the additive should not be easily poisoned by other components in the FCCU, nor should it generate undesirable side reactions.
All of these requirements were achieved in development of COP–NP by use of Intercat’s proprietary formulation and manufacturing techniques.
Experience at refineries
The Marathon consent decree required catalytic additives to be used in five of the seven FCCUs to control NOx emissions. Since Intercat’s COP–NP did not become commercially available until 2004, Marathon conducted its initial consent–decree trials of non–Pt combustion promoters with products from other vendors. Results were successful in that NOx emissions decreased from levels seen when Pt promoters were used, but maintaining adequate afterburn control proved difficult, requiring several times higher additive additions than was desirable.
Following the demonstration period and having agreed with EPA on final flue–gas emissions limits, Marathon began searching for a second–generation non–Pt combustion promoter, one with better promotion activity and at least equivalent NOx reduction capability. By this time, performance results of COP–NP at non–Marathon refineries were just becoming available, some of which appear in Table 1.
In these unit trials, conducted at different US refineries, COP–NP proved itself effective in controlling afterburn and CO emissions while at the same time producing much less NOx than Pt–containing combustion promoters. These trials determined that CO combustion promotion activity and afterburn reduction capability of COP–NP was roughly equivalent to that of Intercat’s COP–375, a 375–ppm Pt–containing promoter. NOx generation by COP–NP, however, was far less than with the Pt–containing promoter.
Marathon agreed to conduct competitive trials at multiple sites to evaluate various non–Pt promoters, selecting three different vendors for these trials.
Based upon the successful results at several other refineries, Marathon first started using COP–NP at its Garyville, La., refinery FCCU in late 2006. After analyzing the data from multiple trials with multiple vendors, Marathon decided to extend use of COP–NP to three additional units. Table 2 shows results from these trials.
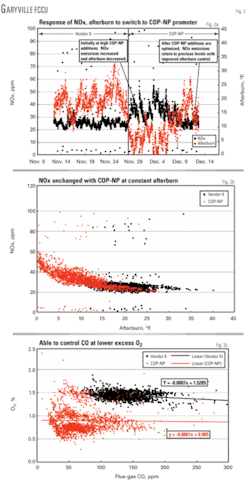
The changeover to COP–NP at each location did not pass without incident, however. When Marathon first began using COP–NP, the unusually high promotion activity of this additive compared with competitive non–Pt promoters was not well understood. COP–NP was initially added at the same rate as Vendor X’s product, the previous non–Pt promoter. This resulted in a large increase in promotion activity in the unit and initially led to a small increase in NOx emissions. Fig. 2a shows this difference between the two additives in effectiveness on NOx emissions and afterburn control at constant addition rates.
As soon as it was realized that COP–NP was significantly more active than Vendor X’s product, the addition rate of COP–NP was gradually reduced. Additions were finally reduced to less than half the rate required for use of Vendor X’s product.
Even at this lower addition rate, afterburn decreased by 48% and CO emissions decreased by 47%. Fig. 2b shows that at a constant degree of afterburn, the NOx emissions with COP–NP were the same as with the previous additive.
Fig. 2c shows an additional benefit of COP–NP use at the Garyville refinery. It was found that CO emissions could be controlled at much lower excess oxygen levels ~ 0.7%) with COP–NP compared with the about 1.5% level previously required. This was of major operational benefit to the FCC in that it relaxed the constraint on the main air blower.
In terms of economic value added, the lower addition rate required with COP–NP resulted in a large reduction in operating expenses. This combined with benefits of the reduced air blower rate and the possibility of being able to increase feed rate as a result provided unanticipated economic benefits and operating flexibility to the Garyville FCCU.
Following successful implementation at the Garyville refinery, Marathon expanded its use of COP–NP to other FCCUs in its system.
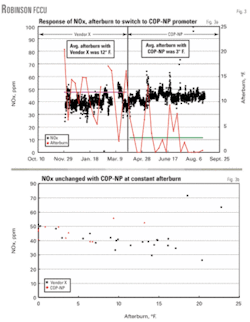
The second Marathon FCCU to use COP–NP was the Robinson, Ill., refinery. This FCCU operates in partial burn with 1.0 to 3.0% CO in the regenerator flue gas. This unit historically used a small amount of Pt COP to control the temperature profile of the bed. NOx emissions would increase dramatically, however, when the unit was operated in full–burn (>300 ppm).
In an effort to minimize emissions, Marathon started using a non–Pt COP manufactured by Vendor X. The required amount of this competitive product was 120 lb/day with NOx emissions averaging 42 ppm (vol) at 12° F. afterburn (Fig. 3a). When COP–NP was used at only 40 lb/day (a threefold reduction in additive additions), however, NOx emissions averaged 46 ppm (vol) and afterburn decreased to an average of only 3° F. A further decrease in additions of COP–NP is possible, which would result in even greater NOx emissions reductions.
This trial shows again that NOx emissions are the same with COP–NP as with the previous competitive product (Fig. 3b) with a threefold reduction in additive additions at constant degrees of afterburn. The conclusion is that COP–NP is a much more efficient and cost–effective additive.
Results of the COP–NP trial at Marathon’s Detroit refinery were much the same as reported for the previous two refineries. This unit had been operating with severe afterburn but had accepted it in order to keep NOx emissions low enough to satisfy the company’s consent–decree emissions requirements. In this case, COP–NP replaced Vendor Y’s non–Pt combustion promoter.
COP–NP reduced afterburn by 19% and CO emissions decreased by 54%. NOx emissions remained essentially unchanged. The required promoter addition rate was reduced by 38% during use of COP–NP vs. vendor Y. Overall, COP–NP was successful in reducing afterburn and CO emissions, without increasing NOx emissions.
The fourth FCC unit in the Marathon system to convert to COP–NP was the St. Paul Park, Minn., refinery. Additions of COP–NP began in October 2008; this unit is just now yielding initial performance results.
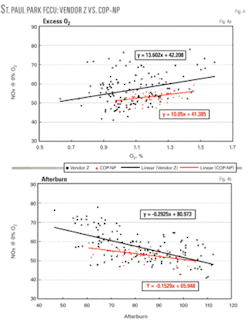
In this case COP–NP replaced Vendor Z’s non–Pt promoter. In addition to the results shown in Table 2, Fig. 4a shows that at the same level of excess O2 in the regenerator, COP–NP generates less NOx. In addition, Fig. 4b shows again that at constant degrees of afterburn, the level of NOx emissions is the same or lower than with the competitor’s additive.
Table 3 summarizes the performance data for COP–NP relative to the historical use of other additives it replaced in each of the Marathon FCC units. The data between these trials are consistent in that they show COP–NP to be more effective in reducing afterburn and CO emissions; more active, resulting in less additive required to be used; and more cost effective than other non–Pt CO combustion promoters that have been used.
Overall, the improved combustion promotion effectiveness of COP–NP has resulted in use of less additive for afterburn and CO emissions control and has led to significant cost savings for each of Marathon’s FCC units.
Based upon the success of COP–NP, Marathon now uses the Intercat non–Pt combustion promoter on six of its seven FCC units. The only unit not using COP–NP continues to use Pt–COP with a backend control technology to manage NOx.
References
- Sexton, J., Joyal, C., and Foley, P., “EPA consent decrees: Progress on FCC Implementation,” NPRA Annual Meeting, Mar. 20, 2007.
- Aru, G.W., “FCC additive demonstrations—Part 1,” PTQ, Q3, 2004.
- Aru, G.W., “FCC additive demonstrations—Part 2,” PTQ, Q4, 2004.
- Radcliff, C., “Reducing FCC unit NOx emissions,” PTQ Catalysis, 2008.
- Evans, M., “Evaluating FCC Flue Gas Emission Control Technologies,” Petroleum Technology Quarterly, first–quarter 2008.
- Peters, A.W., Zhao, X., and Weatherbee, G.W., “The Origin of NOx in the FCC Regenerator,” AM–95–59, National Petroleum Refiners Association, 1995 Annual Meeting.
The authors
Jeffrey Sexton ([email protected]) is the cat cracking technologist for Marathon Oil Co., Findlay, Ohio, responsible for FCC technical and operating performance across Marathon’s seven refineries. He previously worked for UOP in a variety of FCC assignments and has worked on more than 40 different FCC units around the world. Sexton currently chairs the US EPA Consent Decree FCC Technical Team and PSRI Technical Advisory Committee. He holds a BS in chemical engineering from Rose–Hulman Institute of Technology.
Rick Fisher ([email protected]) for the last 4 years served as senior technical service engineer for Intercat. He also worked for 7 years as a process engineer and planning engineer for the Fina/Total/Alon refinery, Big Spring, Tex., and 10 years as a process engineer, plant engineer, and process engineering manager for UDS Sunray, Tex., refinery. Fisher holds a BSc chemical engineering (1988) from the University of Oklahoma, Norman.