Nuclear heat advances oil shale refining in situ
Technological advances in producing and refining liquid fuels in situ using high-temperature heat from nuclear reactors can resolve two major problems—dependence on oil from unstable areas of the world and greenhouse gas emissions—says MIT nuclear engineer Charles W. Forsberg.
In a presentation to the International Congress on Advanced Nuclear Power Plants (ICAPP 08) in June, Forsberg proposed the use of high-temperature heat (nearly 700° C.) from nuclear reactors to refine (underground) hydrocarbon feedstocks such as heavy oils, tar sands, oil shale, and coal to produce light distillates requiring little additional refining to produce gasoline, diesel, and jet fuel. Underground refining also could recover remaining oil in depleted oil fields.
In addition, he said, with major retrofitting at large refineries, nuclear energy also could replace in the mid-term more-costly natural gas in providing high-temperature heat at existing refineries and for producing hydrogen for them in the long term. The high-temperature heat would be used for distillation and thermal cracking—the same processes for which it would be used in underground refining. Several oil companies are looking at nuclear options for heat at refineries and for oil recovery, he said, but retrofitting would be a serious constraint at many refineries. Although nuclear reactors have low operating costs, installation is capital-intensive, Forsberg noted.
While oil supplies 39% of US energy needs, Forsberg said, 149 US oil refineries consume more than 7% of US energy, particularly now that refineries are processing heavier feedstocks requiring more energy to refine. As an extreme case, the production of liquid fuel from coal consumes almost twice the energy value of the liquid fuel produced, he said, making recovery of liquid fuel from coal and some other fossil fuels economically unfeasible with existing technologies if there are constraints on greenhouse gas emissions.
The heavy oil refining trend also implies large increases in carbon dioxide releases per liter of liquid transport fuel produced. These could be greatly reduced by refining in situ, where carbon residue would remain underground, sequestered as carbon solids (coal).
Underground refining
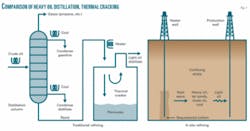
The concept for underground refining is simple, he said. The hydrocarbon deposit is heated to high temperature, and as temperatures increase, volatile hydrocarbons vaporize and move toward recovery wells. They condense in the cooler zones and can then be pumped out of the ground as liquids or vapor.
“This distillation process leaves most impurities behind,” Forsberg said. “While capillary forces can hold liquids in cracks in the rock, gasses easily permeate most reservoirs. As the temperature further increases, heavier hydrocarbons will be thermally cracked to produce lighter volatile hydrocarbons that can be recovered. In effect, heating the underground reservoir duplicates the distillation and thermal cracking processes found in a refinery” (Fig. 1).
This option has become potentially viable because of three technical developments, he said: “precision drilling, underground isolation of geological formations with freeze walls, and the understanding that the slow heating of heavy hydrocarbons (vs. fast heating) increases the yield of light oils while producing a high-carbon solid residue.” He said the high temperatures required are within the current capabilities of high-temperature reactors.
The environmental advantages of in situ refining would be the reduction of toxic heavy metals from the surface environment by leaving them in the ground, avoiding the handling of many carcinogens in the refinery processing of hydrocarbons, and sequestration underground of the carbon as carbon from the thermal cracking process. Sequestration of carbon as solid carbon is known to work, but “the jury is still out for large-scale sequestration of carbon dioxide,” Forsberg said. Any constraints on greenhouse gas releases would provide large economic incentives to use nuclear energy for fuels production.
In addition, it may be possible to undertake hydrocracking by injecting hydrogen into the subsurface while it is being heated, Forsberg said. “However, this option has not been investigated.” Underground hydrocracking could potentially increase liquid yields.
The US as oil exporter
The most technical progress has been made in the recovery of shale oil. Shale oil deposits represent the most concentrated sources of fossil fuels in the world as measured in energy content per unit area. Most deposits are more than 500 ft thick, with some of them more than 2,000 ft thick and parts of the Green River basin yielding more than 2.5 million bbl of oil/acre, he said. By comparison, rich coal deposits in Campbell County, Wyo., yield the energy equivalent of less than a half million barrels per acre of oil.
The US contains the largest oil shale deposits in the world within the Green River Formation in Colorado, Utah, and Wyoming, with 500 billion-1.1 trillion bbl of potentially recoverable shale oil, Forsberg said. “The midpoint estimate is 800 billion bbl, or about three times that of Saudi Arabia.”
“Using these [nuclear] resources for liquid fuel production would potentially enable the United States to become an exporter of oil while sequestering carbon from the refining process underground” as carbon residue, Forsberg told ICAPP.
Traditional recovery
In the traditional process for shale oil recovery, a volume below the retort zone is mined, and staged explosives create rubble of the shale to be retorted. Oxygen is pumped in to burn some of the carbon to produce the required heat, about 480° C.
Traditional processes, however, are expensive, release large amounts of carbon dioxide into the atmosphere, and can produce poor-quality, unstable oil that requires major refining. Initial costs for commercial plants are expected to be $70-95/bbl. With additional industrial experience, costs could drop to $30-40/bbl, Forsberg said.
Shell’s in situ process
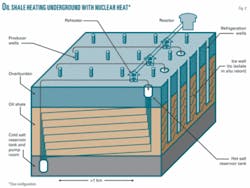
Shell and others have developed new types of in situ retorting, however, that would produce premium shale oil for about $30/bbl. Shell’s in situ conversion process, which involves heating oil shale slowly over many months under chemically reducing conditions and utilizes an ice wall to isolate the in situ retort, is closest to commercial deployment (OGJ, July 10, 2006, p. 18). It has been tested on a small scale and is being scaled up to a precommercial size.
Shell uses electricity for heat—which accounts for about half the total production cost—and requires 15-25 heaters/acre, with the electricity likely created from coal, which is cheaper than gas but environmentally undesirable. The water requirements for the electric power plant also would have a negative environmental impact. Producing 5 million b/d of oil would require 60,000 Mw of electricity.
Compared with traditional processes, it would take 2-3 years of slow heating to 370° C. to release the oil. Higher temperatures could reduce the number of required heaters or decrease the heating time, however.
The nuclear alternative
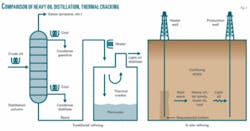
The nuclear option would transfer high-temperature heat from reactors to oil shale via liquid-metal or liquid-salt heat transport loops (Fig. 2). The distances from reactor to wellhead are short, making heat transport practical.
The process offers several advantages: The energy requirements would be reduced by a factor of 2 or more from systems using electricity, with expensive electricity replaced by lower-cost thermal energy, Forsberg said. With electric heating options, heat is converted to electricity and converted back to heat. The direct use of heat from a nuclear reactor avoids these conversion losses. The use of an intermediate heat transport loop also allows recovery of some of the heat after oil extraction, and the heat can be reused to partly heat the next oil-bearing rock. This can reduce heat requirements by a factor of 2 or more relative to electric heat or combustion heating of the oil-bearing rock.
And because electricity is not used, carbon dioxide emissions are avoided along with the need for excessive water use. In addition, with nuclear heat, all of the products are recovered and none need be burned to provide heat. A light, stable crude oil is produced that leaves impurities in the ground and requires relatively little refining. A high-temperature nuclear reactor can directly produce the necessary heat. “Good economics requires long-term base-load operations,” said Forsberg.
Underground refining should be applicable to a wide variety of fossil deposits and is also effective in recovering oil from exhausted fields in which more than half the original oil remains after a field is abandoned. Although oil is held in place by strong capillary forces, when it is vaporized, it will flow and can be recovered even through tight capillaries.
“Technical challenges associated with nuclear energy use for oil shale production include the selection of the appropriate coolant-materials combinations for the heat transfer loops with the development of the startup-shutdown procedures,” said Forsberg who is a member of the Nuclear Science and Engineering Department at the Massachusetts Institute of Technology. “About 12 Gw+ of high-temperature heat would be required to produce a million barrels of oil per day, with required reactor temperatures near 700° C.” Like all other oil recovery technologies, the applicability of the technology will depend upon the local geology. Only field testing can determine the capabilities and limits of the technology.
Heating oil sands and depleted oil fields differs somewhat from heating oil shale or coal. Heating oil sands and depleted fields lowers the viscosity and surface tension of the oil and allows some fraction of the oil to flow as a liquid to the production wells. Residual oil further heated is either vaporized and condenses near the cooler production wells or is thermally cracked into more volatile hydrocarbons. These applications likely will require less thermal energy.