Modified cycles, adsorbents improve gas treatment, increase mol-sieve life
Natural gas treatment often uses adsorbents for contaminant removal. Special materials, such as molecular sieves, remove sulfur-containing impurities like mercaptans, as well as water vapor. Such operation may be prone to problems, particularly during regeneration of these molecular sieves. The transient thermal waves produced during regeneration, for example, may create unwanted byproducts like COS.
Improper regeneration may ultimately reduce product quality if the temperature is too low or time is too short. On the other hand, excessive temperatures or heating times may reduce adsorbent life or cause adsorbate decomposition (or coking) in some instances.
Modified cycling, as well as additional adsorbents (such as alumina), can reduce degradation of molecular sieves. Such additional adsorbents also allow targeting and removal of other contaminants, including oxygenates like methanol that may promote undesirable side reactions on valuable molecular sieves.
This article will discuss effective compound-bed configurations and process techniques to address problems sometimes encountered with conventional mole sieve treatment.
ExxonMobil Corp. operates dozens of gas-treatment plants worldwide and is committed to using modern, advanced materials, as well as lowering regeneration-energy requirements, thereby reducing emissions.
Improving performance
Molecular-sieve (MS) adsorbents have become widespread for removing contaminant from natural gas. Over the last 3-4 decades, they have proven suitable and economic in reducing contaminant levels during gas processing. With increasing treatment requirements, however, including more stringent product and effluent specifications, greater plant size, and increasingly difficult feedstocks, there is considerable incentive to improve MS performance.
Simultaneously, decades-long experience from operating plants has begun to reveal limitations in conventional use of these materials. With guidance from the past, MS end-users and manufacturers are developing effective strategies for enhancing use of these materials and maximizing their potential.
ExxonMobil has been involved in adsorption and adsorbent use for at least a half-a-century, with both operating experience and ongoing MS and adsorbent development programs. Relevant experience includes gas treatment at several locations worldwide conducted over the entire gamut of operating conditions. It is one of the largest users of adsorbents supplied by vendors and maintains its own internal programs and research into new adsorbent materials and chemistry.
This unique combination of breadth of operating experience and depth of in-house research capability has allowed ExxonMobil to approach the problem of optimizing adsorbent performance from a unique vantage point.
Key limitations exist in conventional MS operating practice, including deactivation via exposure to certain contaminants, suboptimal regeneration (e.g., refluxing), unwanted byproducts during regeneration, and lowered adsorbent life due to increased regeneration frequency and severity. The company’s strategy addresses these limitations using:
- Enhanced adsorbents including structured adsorbents and adsorbent combinations (compound-beds).
- Modified thermal or pressure cycles including variable-frequency cycling.
- Modified partial desorption regeneration,1 or combinations thereof. The company is seeking patents on some of these combinations and exploring more selective adsorbents to reduce hydrocarbon loss and regeneration energy requirements.
Delaying degradation
The desire to reduce adsorbent fill cost and to increase the lifetime of solid-bed dehydrators has led to consideration of alternative adsorbents, including their use with compound beds. Compound (or “mixed”) beds consist of two or more layers of different types of MS for water and mercaptan sulfurs, for example.
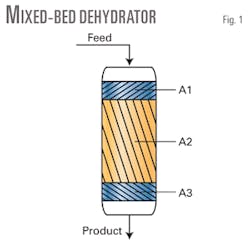
Fig. 1 shows such a compound bed of adsorbents, where A1, A2, and A3 represent different solid materials. Adsorbents can be MS, such as 4A (often used for dehydration) or 13X (used for removal of larger molecules), or other zeolitic adsorbents. Additional layers of specific adsorbents may also be used to remove trace mercury, for example, if the treated gas is to be fed to an LNG plant.
Additional layers can also offer significant protection of sensitive molecular sieves from both contaminants and damaging conditions that might result from upsets during the feed cycle or refluxing during the regeneration cycle. The air-separation industry often uses compound beds as pre-purifiers.2
Typically, contaminated feed gas enters from the top of the bed during the adsorption cycle. As the bed adsorbs contaminants over the course of the service cycle, it ultimately must be taken off-line before it exceeds treated-gas specifications. Usually, another bed is placed in service, and the spent bed regenerated.
During the regeneration cycle, dry, heated gas flows in from the bottom of the bed and progresses up through the packed bed. A “thermal wave” moves up through the bed as contaminants flow out. In general, there is a lag between arrival of the thermal front and arrival of the mass transfer front. This can create conditions for unwanted side reactions.
The contaminant-saturated regenerated gas is usually cooled to condense water and heavy hydrocarbons. If other contaminants are present, the regeneration stream must be treated in some manner. For example, the stream may be washed with physical solvent to absorb sulfur compounds, so that the treated gas may be used for fuel.
The unsteady nature of the regeneration process generates a “peak” of contaminants. Generally, the solvent system must be operated in a way to provide a steady concentration of contaminants to the next process (e.g., sulfur-recovery unit).
The addition of activated alumina (AA) to the top of these compound beds of MS protects the MS from unexpected liquid (hydrocarbon, brine, glycol, or condensed water) carryover into the bed from upstream separation facilities sometimes as aerosols. Well-designed separation equipment upstream of the MS bed can reduce such fouling.
AA can also protect the MS from “refluxing,” when water condenses in the upper, cooler part of the bed during early stages of regeneration (discussed below). AA, because of its higher water capacity, also helps ensure that the heavy compound (e.g., mercaptan) removal specification will be met by reduction of the chance of water breakthrough from a compound bed consisting of 4A MS and 13X MS, for example.
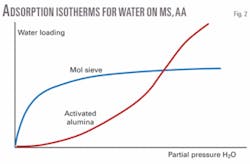
Even a relatively small amount of water breakthrough into the 13X MS will reduce the mercaptan-removal capacity substantially because of the adsorbent’s greater affinity for the more polar compound. 13X capacity cannot be restored until the water is desorbed. There are several benefits of using an AA/MS compound bed including lower overall adsorbent cost and higher resistance to liquid upsets and liquid carryover (described above). AA can also have a higher equilibrium capacity than MS when the feed gas is near saturation, as shown by the isotherms in Fig. 2.
Regeneration temperatures required for AA are furthermore lower than those of MS, thus lowering the heat requirement to desorb the water in the AA relative to MS. This lowered energy requirement translates to savings of steam or fuel gas, depending on how the regeneration gas is heated. Others in industry have recognized the benefits of compound beds for natural gas treatment, and compound beds of AA/MS have been used in commercial natural gas dryers at several installations for decades.2-7
Sweet gas dehydration
A particularly undesirable limitation of conventional MS use occurs if there is uncontrolled water condensation during regeneration. At the start of the regeneration cycle, the MS at the top of the bed is already near its maximum water-adsorption capacity (for that temperature and partial pressure of water) because it has been in drying service cycle for some time. The regeneration thermal wave moving up from the bottom of the vessel drives off water from the heated adsorbent and carries it in the gas phase.
Hot gas moving up through the cooler part of the bed cools and may reach the point of water saturation, particularly if the regeneration is at high pressure. Since the remaining incremental capacity of the MS is small (for fixed temperature) the water may condense, which can be damaging to the binder of the sieve particles. The advancing thermal wave can then vaporize the condensed water, potentially causing pressure build-up in the MS particles. If the rate of temperature rise is too rapid, the particles may be damaged. Also, if the bed is internally insulated, it may be possible for liquid water to get behind the insulation. In sour service, this could create corrosion issues.
In the worst cases, liquid water may condense, start flowing down the bed, and be revaporized by the advancing regeneration thermal wave, a phenomenon known as “refluxing.” The ultimate result is often a doughnut-shaped region of powdered MS material at the top of the adsorbent bed. AA, on the other hand, generally resists disintegration by liquid water,8 although its porosity may decrease under these conditions.
It has been suggested to reduce the effect of refluxing by use of alternate types of molecular sieves that are mechanically stronger and more resistant to disintegration.9 Like the use of AA, however, they do not actually address the root cause of the problem.
One way of reducing the chance of refluxing is more slowly to ramp the temperature of the regeneration gas over the course of the cycle. This may lengthen the regeneration step to achieve the same level of water removal. This milder regeneration scheme, however, is generally preferable to premature aging and unscheduled changeout of the MS.
In many cases, the regeneration heater is immediately activated to maximum temperature (typically around 315° C.) and held there throughout the regeneration step. While this does minimize the time for regeneration, it also maximizes the severity of conditions for the adsorbent, as well as such ancillary equipment as switching valves, support screens, and internal insulation (if present).
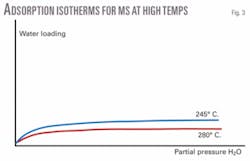
In the simplest cases, reducing the final regeneration temperature can lengthen the life of the adsorbent. While a small amount of net adsorption capacity may be lost (Fig. 3) and the cycle time may be increased slightly, the decreased rate of water desorption will decrease the supersaturation of the gas at the top of the bed, reducing the chance for reflux.
Testing the method at one installation, ExxonMobil found that a reduction of up to 35° C. in regeneration temperature was possible (245° C. vs. 280° C.), with no apparent immediate effect on the drying cycle. This also yields the benefits of reducing undesirable side reactions and total energy requirement. One possible means of countering the increased regeneration cycle time would be to increase the hot-gas flow rate. Velocities, however, are limited to those that will not cause lifting of the bed.
The addition of heat to the top end of the vessel at the start of regeneration could mitigate refluxing. This may not necessarily fully protect the MS in the middle (radial sense) of the upper portion of the bed, but it will address the area of greatest thermal inertia and could prevent formation of the ring of disintegrated MS that is sometimes observed when a spent bed is opened. One must avoid water condensation on downstream carbon steel piping, however, when it is exposed to sour gas.
Even when refluxing does not occur, molecular sieves are subject to hydrothermal aging during normal operation. Because the loss of activity can be substantial after a 3-5-year lifetime, the beds are generally designed for end-of-run (EOR) conditions. Thus, at start-of-run (SOR) adsorbent conditions, extra capacity in the design can be used to advantage.
One alternative is simply to run for a longer period in drying service, rather than for a fixed (EOR) time. Another possibility is to utilize lower regeneration temperatures at SOR conditions. Some residual capacity may be lost during these cycles, but the severity of regeneration is reduced. This scheme will increase the total number of cycles achieved before EOR conditions are reached. Of course, regeneration temperatures may have to be adjusted upward over time to compensate for the hydrothermal aging that eventually occurs.
Ultimately, there is a trade off between milder regeneration conditions, which reduce operating expenditures (OPEX; through decreased material changeout frequency and reduced energy) and increasing capital expenditures (CAPEX). Because lower regeneration temperatures reduce net capacity slightly, a corresponding increase in bed size is needed to offset this, increasing CAPEX.
If the added volume of material requires an additional dehydration vessel, then the required CAPEX clearly exceeds any incremental OPEX savings obtained through energy savings and increased adsorbent life. For existing installations at SOR adsorbent conditions, lagniappe in the design can be used to advantage, since facilities are generally designed for EOR conditions, when adsorbent activity is decreased after many cycles.
Use of lower temperatures at SOR conditions can increase the total number of cycles achieved before EOR conditions are reached.
If only dehydration of a sweet gas stream is required, a common means of avoiding the negative effects of gas-phase impurities (such as benzene, toluene, and xylene) is to use small-pore zeolites (3A or 4A).10 Mercaptans, heavy hydrocarbons, and other large molecules cannot fit into the small pores. Thus, they are not present in large concentration when the thermal wave arrives. Thus, degradation via coking is not generally an issue (though hydrothermal aging may be).
Sour service
It has been long observed4 11 that CO2 and H2S can react to form COS according to H2S + CO2 → H2O + COS.
This reaction is particularly prone to occur at the elevated temperatures of regeneration.
For dehydration-only service (i.e., no H2S removal), it is possible to use a small-pore zeolite (3A) MS in order to exclude the reactants from the pore space.12 Alternatively, it has been shown that 5A MS catalyzes COS formation to a lesser degree than 4 A MS because it is less basic than 4A MS.11
HC, mercaptan removal
In some cases, it is desirable to remove heavy hydrocarbons from a sour-gas stream. ExxonMobil has successfully used silica gel in this application to reduce heavy hydrocarbons going to a solvent-based acid gas removal process.13 Interestingly, regeneration temperatures had to be lowered to reduce the effects of sulfidation in the regeneration gas heater coils.
Removing mercaptans from gas typically employs larger pore-size molecular sieves (like 13X). Heavy hydrocarbons (like BTX) and other impurities (like methanol) will coadsorb with mercaptans. Consequently, it is possible for these compounds to crack and form coke if the thermal wave “outruns” them during regeneration.
Some reports have also noted the decomposition of mercaptans on 13X.14 Thus, if the impurities are not desorbed and flushed out before the thermal wave arrives, they may react in some fashion, possibly catalyzed by the solid itself. Again, reduced regeneration temperatures will tend to reduce these undesirable side reactions.
Another means to reduce decomposition reactions is to ramp the temperature more slowly during initial stages of regeneration. This allows more time for the molecules to desorb, diffuse, and flow out of the bed before the high temperature front arrives. A variation of this theme is to bring the temperature up to some intermediate value and hold it there for some time, then ramp to the final temperature. Ramping, or holding at intermediate temperatures, also reduces the thermal stress placed on the system but increases the regeneration cycle time.
Of course, any modification to regeneration cycles requires agreement from operations, as adjustment of the regeneration stream cleanup system may also be needed. It is ideal to have the material vendor in agreement with the changes, but this may not always occur.
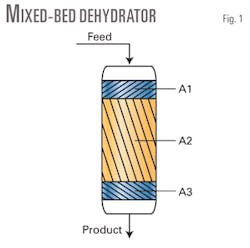
Compound beds
To minimize adverse effects of the cracking of heavy components, we suggest using compound beds (i.e., MS and desiccant), including modified alumina in the product zone, for example in Zone A3 in Fig. 1. ExxonMobil’s studies indicate that, by doing so, one could use a regeneration stream with lower temperature and also a regeneration stream that contains a small amount of water vapor that may suppress coking reactions.14
Adding water vapor to the regeneration stream for a short period may act to desorb heavy molecules (BTX and mercaptans) before the thermal wave arrives. In effect, it may be a modified desorptive,1 or displacement type of regeneration.15 Use of alumina controls the rate at which water is added to the regeneration stream.
Figs. 4 and 5 exemplify the advantages of our approach compared with using only MS (the conventional approach). These figures were generated with the aid of an in-house computational simulator of cyclic adsorption processes, which derives from data from ExxonMobil’s upstream, downstream, and chemical companies.
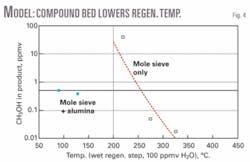
Fig. 4 illustrates the conclusion that use of AA in the bottom layer (“mole sieve + alumina” points on the graph) allows for a substantially lower regeneration temperature than MS alone, while still meeting a <1 ppm methanol specification in the product stream after a typical service time. The product spec will not be met for the mole-sieve-only case for regeneration temperatures less than 200° C. (Decomposition effects were not considered.)
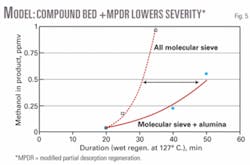
Fig. 5 shows the potential effect of allowing wet regeneration to go for too long (total regeneration cycle time is 124 min). If residual water remains on the MS, product quality will suffer. This is particularly true for the molecular-sieve-only case.
Alternative adsorbents
ExxonMobil is also testing an entirely new class of adsorbents for natural gas purification. These adsorbents are mixed-metal oxide catalysts with mixtures of alumina or silica substrates. Our tests have determined that these materials have reasonable capacity for the principal contaminants found in natural gas.
Experiments with both fresh and sulfided forms of these catalysts indicate that it may be possible to recycle catalysts after their use in refining operations such as hydrotreating and hydrofining. (The material would likely require coke removal, however, using a standard decoking procedure before its new life as an adsorbent.)
The spent catalysts also show reduced uptake of nonsulfurous components (i.e., heavier hydrocarbons). This feature makes these catalyst materials attractive, compared with conventional natural-gas-treatment molecular sieves. The latter are generally indiscriminate in the uptake of all heavy components of natural gas.
Selective adsorbents would not remove as many valuable heavy hydrocarbons from the feed stream. These valuable molecules would then not end up in the sulfur-recovery unit (SRU) where they consume a lot of air (and, if not completely destroyed, soot the SRU catalyst).
ExxonMobil’s test results have also shown the possibility of using regeneration techniques of reduced severity (temperature or duration) with the mixed-metal oxide catalysts as adsorbents. This reduces the thermal energy (steam or direct fire) required to heat the regeneration gas, thereby reducing emissions. Reduced severity of regeneration also prolongs adsorbent life. Taking this to the extreme, modified pressure swing cycles can eliminate hydrothermal effects altogether.
We have employed the analogy between adsorption from liquids and adsorption from unsaturated vapors to help quantify the potential effectiveness of these new adsorbents. In our experiments, breakthrough curves of sulfur-containing compounds were obtained from contaminated liquid hydrocarbons passing through a bed of cobalt molybdenum hydrotreating catalyst. We observed early breakthrough of thiophenes; these compounds, however, are not generally present in appreciable concentration in gas streams.
Liquid-phase adsorption is well known to possess limitations due to lower diffusivities (lower mass transfer) and higher densities, which promote capacity-reducing adsorption non-idealities. The loading and uptake observed in our liquid-phase experiments indicate good capacity using the analogy of adsorption from liquids and adsorption from gases. The analogy shows that enhancement of adsorption from the binary gas vs. that from binary liquid is possible when the mixed-gas isotherms are compared. The ratio of gas-phase mixed Langmuir loading and liquid-phase Langmuir surface excess can be derived as shown in the accompanying box.16
Gas-phase testing of these adsorbents has yet to be done. We recognize, however, that these materials are used in hydrogenating services and that they may become sulfided during regeneration with sour gas and could also require special handling. Given that the materials themselves are essentially free of charge (spent catalyst that would otherwise require disposal) and given the potential for much greater selectivity toward sulfur compounds, these materials warrant further evaluation.
Acknowledgment
This article is dedicated to the memory of our friend and colleague Francis Wu.
References
Based on a presentation to the Laurance Reid Gas Conditioning Conference, Norman, Okla., Feb. 25-27, 2008.
The authors
Scott Northrop (scott.northrop @exxonmobil.com) is group lead for gas treating and sulfur recovery in the gas and facilities division of ExxonMobil’s Upstream Research Co., Houston. His group is responsible for developing and commercializing gas separation and sulfur-related technologies. He has more than 20 years’ experience in various parts of the upstream sector. Northrop received his BS in chemical engineering at Washington University, St. Louis, and his MS and PhD degrees in chemical engineering from the California Institute of Technology. He is active in the Gas Processors Association, serving on Technical Section F, Subgroup 1 (Thermodynamic data), and on the Editorial Review Board for the Gas Processors Suppliers Association Databook. He also serves on the board of directors of Alberta Sulfur Research Ltd.
Narasimhan Sundaram ([email protected]) works for ExxonMobil Research and Engineering, Fairfax, responsible for adsorption processes supporting upstream and downstream operations. He is leader of an EMRE community for development of innovative separations that recently commercialized rapid cycle pressure swing adsorption. Sundaram received a BTech from IIT, New Delhi, and his PhD (1990) in chemical engineering from Purdue University, W. Lafayette, Ind. He has been active in adsorption process engineering for more than 22 years.